Neurotransmitter abnormalities and response to supplementation in SPG11
- PMID: 22749184
- PMCID: PMC3517733
- DOI: 10.1016/j.ymgme.2012.05.020
Neurotransmitter abnormalities and response to supplementation in SPG11
Abstract
Objective: To report the detection of secondary neurotransmitter abnormalities in a group of SPG11 patients and describe treatment with l-dopa/carbidopa and sapropterin.
Design: Case reports.
Setting: National Institutes of Health in the Undiagnosed Disease Program; Children's National Medical Center in the Myelin Disorders Bioregistry Program.
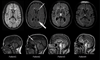
Patients: Four SPG11 patients with a clinical picture of progressive spastic paraparesis complicated by extrapyramidal symptoms and maculopathy.
Interventions: L-Dopa/carbidopa and sapropterin.
Results: 3/4 patients presented secondary neurotransmitter abnormalities; 4/4 partially responded to L-dopa as well as sapropterin.
Conclusions: In the SPG11 patient with extrapyramidal symptoms, a trial of L-dopa/carbidopa and sapropterin and/or evaluation of cerebrospinal fluid neurotransmitters should be considered.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Samaranch L, Riverol M, Masdeu JC, et al. SPG11 compound mutations in spastic paraparesis with thin corpus callosum. Neurology. 2008;71:332–336. - PubMed
-
- de Bot ST, van de Warrenburg BP, Kremer HP, Willemsen MA. Child neurology: hereditary spastic paraplegia in children. Neurology. 75:e75–e79. - PubMed
-
- Guidubaldi A, Piano C, Santorelli FM, et al. Novel mutations in SPG11 cause hereditary spastic paraplegia associated with early-onset levodopa-responsive Parkinsonism. Mov Disord. 2011;26:553–556. - PubMed
-
- Anheim M, Lagier-Tourenne C, Stevanin G, et al. SPG11 spastic paraplegia. A new cause of juvenile parkinsonism. J Neurol. 2009;256:104–108. - PubMed

