Structural insights for engineering binding proteins based on non-antibody scaffolds
- PMID: 22749196
- PMCID: PMC3423532
- DOI: 10.1016/j.sbi.2012.06.001
Structural insights for engineering binding proteins based on non-antibody scaffolds
Abstract
Engineered binding proteins derived from non-antibody scaffolds constitute an increasingly prominent class of reagents in both research and therapeutic applications. The growing number of crystal structures of these 'alternative' scaffold-based binding proteins in complex with their targets illustrate the mechanisms of molecular recognition that are common among these systems and those unique to each. This information is useful for critically assessing and improving/expanding engineering strategies. Furthermore, the structural features of these synthetic proteins produced under tightly controlled, directed evolution deepen our understanding of the underlying principles governing molecular recognition.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
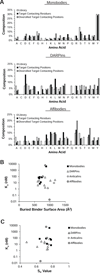
References
-
- Skerra A. Alternative non-antibody scaffolds for molecular recognition. Curr Opin Biotechnol. 2007;18:295–304. - PubMed
-
- Koide S. Design and engineering of synthetic binding proteins using nonantibody scaffolds. In: Park SJ, Cochran JR, editors. Protein Engineering and Design. CRC Press; 2010. pp. 109–130.
-
- Koide S, Koide A, Lipovsek D. Target-binding proteins based on the 10th human fibronectin type III domain ((1)Fn3) Methods Enzymol. 2012;503:135–156. - PubMed
-
-
Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, Grutter MG, Pluckthun A. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol. 2004;22:575–582. The authors report the first structure of a DARPin in complex with its target. This structure exemplifies the characteristic binding mode of DARPins. This structure is the most prominent example of the enrichment of aromatic amino acids in DARPin binding sites with 71% of interface surface contributed by aromatic amino acids.
-
-
- Veesler D, Dreier B, Blangy S, Lichiere J, Tremblay D, Moineau S, Spinelli S, Tegoni M, Pluckthun A, Campanacci V, et al. Crystal structure and function of a DARPin neutralizing inhibitor of lactococcal phage TP901-1: comparison of DARPin and camelid VHH binding mode. J Biol Chem. 2009;284:30718–30726. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

