Photoacoustic flow cytometry
- PMID: 22749928
- PMCID: PMC4799719
- DOI: 10.1016/j.ymeth.2012.06.009
Photoacoustic flow cytometry
Abstract
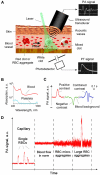
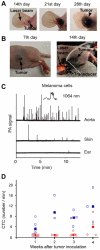
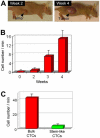
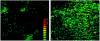
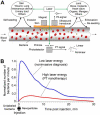
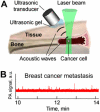
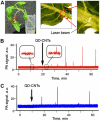
Conventional flow cytometry using scattering and fluorescent detection methods has been a fundamental tool of biological discoveries for many years. Invasive extraction of cells from a living organism, however, may lead to changes in cell properties and prevents the long-term study of cells in their native environment. Here, we summarize recent advances of new generation flow cytometry for in vivo noninvasive label-free or targeted detection of cells in blood, lymph, bone, cerebral and plant vasculatures using photoacoustic (PA) detection techniques, multispectral high-pulse-repetition-rate lasers, tunable ultrasharp (up to 0.8 nm) rainbow plasmonic nanoprobes, positive and negative PA contrasts, in vivo magnetic enrichment, time-of-flight cell velocity measurement, PA spectral analysis, and integration of PA, photothermal (PT), fluorescent, and Raman methods. Unique applications of this tool are reviewed with a focus on ultrasensitive detection of normal blood cells at different functional states (e.g., apoptotic and necrotic) and rare abnormal cells including circulating tumor cells (CTCs), cancer stem cells, pathogens, clots, sickle cells as well as pharmokinetics of nanoparticles, dyes, microbubbles and drug nanocarriers. Using this tool we discovered that palpation, biopsy, or surgery can enhance CTC release from primary tumors, increasing the risk of metastasis. The novel fluctuation flow cytometry provided the opportunity for the dynamic study of blood rheology including red blood cell aggregation and clot formation in different medical conditions (e.g., blood disorders, cancer, or surgery). Theranostics, as a combination of PA diagnosis and PT nanobubble-amplified multiplex therapy, was used for eradication of CTCs, purging of infected blood, and thrombolysis of clots using PA guidance to control therapy efficiency. In vivo flow cytometry using a portable fiber-based devices can provide a breakthrough platform for early diagnosis of cancer, infection and cardiovascular disorders with a potential to inhibit, if not prevent, metastasis, sepsis, and strokes or heart attack by well-timed personalized therapy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures









References
-
- Shapiro HM. Practical Flow Cytometry. fourth ed. Wiley-Liss; New York: 2003.
-
- Sack U, Tárnok A, Rothe G, editors. Cellular Diagnostics: Basic Principles, Methods and Clinical Applications of Flow Cytometry. Karger; Basel, Freiburg, Paris: 2008.
-
- Zharov VP, Galanzha EI, Tuchin VV. Proc. SPIE. 2004;5320:256–263.
-
- Galanzha EI, Tuchin VV, Chowdhury P, Zharov VP. Proc. SPIE. 2004;5474:204–214.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

