Effectiveness of barcoding for reducing patient specimen and laboratory testing identification errors: a Laboratory Medicine Best Practices systematic review and meta-analysis
- PMID: 22750145
- PMCID: PMC4518452
- DOI: 10.1016/j.clinbiochem.2012.06.019
Effectiveness of barcoding for reducing patient specimen and laboratory testing identification errors: a Laboratory Medicine Best Practices systematic review and meta-analysis
Abstract
Objectives: This is the first systematic review of the effectiveness of barcoding practices for reducing patient specimen and laboratory testing identification errors.
Design and methods: The CDC-funded Laboratory Medicine Best Practices Initiative systematic review methods for quality improvement practices were used.
Results: A total of 17 observational studies reporting on barcoding systems are included in the body of evidence; 10 for patient specimens and 7 for point-of-care testing. All 17 studies favored barcoding, with meta-analysis mean odds ratios for barcoding systems of 4.39 (95% CI: 3.05-6.32) and for point-of-care testing of 5.93 (95% CI: 5.28-6.67).
Conclusions: Barcoding is effective for reducing patient specimen and laboratory testing identification errors in diverse hospital settings and is recommended as an evidence-based "best practice." The overall strength of evidence rating is high and the effect size rating is substantial. Unpublished studies made an important contribution comprising almost half of the body of evidence.
Copyright © 2012 The Canadian Society of Clinical Chemists. All rights reserved.
Figures
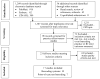


Comment in
-
Evidence in action; commentary.Clin Biochem. 2012 Sep;45(13-14):1033-5. doi: 10.1016/j.clinbiochem.2012.07.102. Clin Biochem. 2012. PMID: 22963709 No abstract available.
References
-
- Kohn LT, Corrigan J, Donaldson MS. To err is human : building a safer health system. National Academy Press; Washington, D.C: 2000. - PubMed
-
- Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–698. - PubMed
-
- Makary MA, Epstein J, Pronovost PJ, Millman EA, Hartmann EC, Freischlag JA. Surgical specimen identification errors: a new measure of quality in surgical care. Surgery. 2007;141:450–455. - PubMed
-
- Christenson RH, Snyder SR, Shaw CS, Derzon JH, Black RS, Mass D, Epner P, Favoretto AM, Liebow EB. Laboratory medicine best practices: systematic evidence review and evaluation methods for quality improvement. Clin Chem. 2011;57:816–825. - PubMed
-
-
CLIA Subpart K - Quality Systems for Nonwaived Testing - General Laboratory Systems - Preanalytic Systems - §493.1242 Standard: Specimen submission, handling, and referral Federal Register, 42 CFR 493.1242.
-
APPENDIX C. LMBP Barcoding Systematic Review Eligible Studies
Barcoding Systems
Included studies
Published
-
- Bologna LJ, Mutter M. Life after phlebotomy deployment: Reducing major patient and specimen identification errors. Journal of healthcare information management : JHIM. 2002;16:65–70. - PubMed
-
- Brown JE, Smith N, Sherfy BR. Decreasing mislabeled laboratory specimens using barcode technology and bedside printers. Journal of nursing care quality. 2010 - PubMed
-
- Hayden RT, Patterson DJ, Jay DW, Cross C, Dotson P, Possel RE, et al. Computer-assisted bar-coding system significantly reduces clinical laboratory specimen identification errors in a pediatric oncology hospital. The Journal of pediatrics. 2008;152:219–24. - PubMed
-
- Hill PM, Mareiniss D, Murphy P, Gardner H, Hsieh YH, Levy F, et al. Significant reduction of laboratory specimen labeling errors by implementation of an electronic ordering system paired with a bar-code specimen labeling process. Annals of emergency medicine. 2010;56:630–6. - PubMed
-
- Killeen JP, Chan TC, Jones K, Guss DA. Impact of bar coding technology and computerized physician order entry on reducing laboratory specimen misidentification errors in the emergency department. Annual Meeting of the Society for Academic Emergency Medicine; New York City. 2005; p. 49.
Unpublished
-
- Heng J, Lyndon B. Johnson General Hospital; 2009.
-
- Schmidt R. University of Washington, School of Medicine; 2009.
-
-
Large academic medical center, Southern CA; 2009.
-
Excluded studies
Published
-
- Fabbretti G. Risk management: Correct patient and specimen identification in a surgical pathology laboratory. The experience of infermi hospital, rimini, italy. Pathologica. 2010;102:96–101. - PubMed
-
- Sandler SG, Langeberg A, Dohnalek L. Bar code technology improves positive patient identification and transfusion safety. Developments in biologicals. 2005;120:19–24. - PubMed
-
- Turner CL, Casbard AC, Murphy MF. Barcode technology: Its role in increasing the safety of blood transfusion. Transfusion. 2003;43:1200–9. - PubMed
Unpublished
-
- Senn C, Bormann P. University of Minnesota Medical Center Fairview; 2009.
Point-of-Care Test Barcoding
Included studies
Published
-
- Colard DR. Reduction of patient identification errors using technology. Point of Care. 2005;4:61–3.
-
- Rao AC, Burke DA, Dighe AS. Implementation of bar coded wristbands in a large academic medical center: Impact on point of care error rates. Point of Care. 2005;4:119–22.
Unpublished
-
- Schuerch C. Geisinger Medical Center; 2009.
-
- Jarnot J, Weber A. Kenmore Mercy Hospital. 2011.
-
- Jarnot J, Weber A. Mercy Hospital of Buffalo; 2011.
-
- Jarnot J, Weber A. Sisters of Charity Hospital Buffalo; 2011.
-
- Jarnot J, Weber A. Sisters of Charity Hospital St. Joseph’s Campus; 2011.
Excluded studies
Published
-
- Nichols JH, Bartholomew C, Brunton M, Cintron C, Elliott S, McGirr J, et al. Reducing medical errors through barcoding at the point of care. Clinical leadership & management review : the journal of CLMA. 2004;18:328–34. - PubMed
Unpublished
-
-
Midwest Academic Medical Center, Minnesota; 2009.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

