Deactivation of hepatic stellate cells during liver fibrosis resolution in mice
- PMID: 22750464
- PMCID: PMC3848328
- DOI: 10.1053/j.gastro.2012.06.036
Deactivation of hepatic stellate cells during liver fibrosis resolution in mice
Abstract
Background & aims: Activated hepatic stellate cells (HSCs), the main fibrogenic cell type in the liver, undergo apoptosis after cessation of liver injury, which contributes to resolution of fibrosis. In this study, we investigated whether HSC deactivation constitutes an additional mechanism of liver fibrosis resolution.
Methods: HSC activation and deactivation were investigated by single-cell PCR and genetic tracking in transgenic mice that expressed a tamoxifen-inducible CreER under control of the endogenous vimentin promoter (Vimentin-CreER).
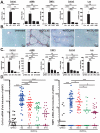
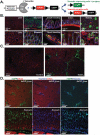
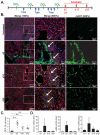
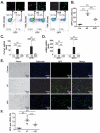
Results: Single-cell quantitative polymerase chain reaction demonstrated activation of almost the entire HSC population in fibrotic livers, and a gradual decrease of HSC activation during fibrosis resolution, indicating deactivation of HSCs. Vimentin-CreER marked activated HSCs, demonstrated by a 6- to 16-fold induction of a membrane-bound green fluorescent protein (mGFP) Cre-reporter after injection of carbon tetrachloride, in liver and isolated HSCs, and a shift in localization of mGFP-marked HSCs from peri-sinusoidal to fibrotic septa. Tracking of mGFP-positive HSCs revealed the persistence of 40%-45% of mGFP expression in livers and isolated HSCs 30-45 days after carbon tetrachloride was no longer administered, despite normalization of fibrogenesis parameters; these findings confirm reversal of HSC activation. After fibrosis resolution, mGFP expression was observed again in desmin-positive peri-sinusoidal HSCs; no mGFP expression was detected in hepatocytes or cholangiocytes, excluding mesenchymal-epithelial transition. Notably, reverted HSCs remained in a primed state, with higher levels of responsiveness to fibrogenic stimuli.
Conclusions: In mice, reversal of HSC activation contributes to termination of fibrogenesis during fibrosis resolution, but results in higher responsiveness of reverted HSCs to recurring fibrogenic stimulation.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Standing down the guard: stellate cells leave quietly.Gastroenterology. 2012 Oct;143(4):890-2. doi: 10.1053/j.gastro.2012.08.014. Epub 2012 Aug 20. Gastroenterology. 2012. PMID: 22917861 No abstract available.
-
Reversion of hepatic stellate cell to a quiescent phenotype: From myth to reality?J Hepatol. 2013 Aug;59(2):383-6. doi: 10.1016/j.jhep.2013.03.031. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567083 No abstract available.
References
-
- Kluwe J, Wongsiriroj N, Troeger JS, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–8. - PubMed
-
- Morcos SH, Khayyal MT, Mansour MM, et al. Reversal of hepatic fibrosis after praziquantel therapy of murine schistosomiasis. Am J Trop Med Hyg. 1985;34:314–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

