Impact of screening test performance and cost on mortality reduction and cost-effectiveness of multimodal ovarian cancer screening
- PMID: 22750949
- PMCID: PMC3729263
- DOI: 10.1158/1940-6207.CAPR-11-0468
Impact of screening test performance and cost on mortality reduction and cost-effectiveness of multimodal ovarian cancer screening
Abstract
Ongoing ovarian cancer screening trials are investigating the efficacy of a two-step screening strategy using currently available blood and imaging tests [CA125 and transvaginal sonography (TVS)]. Concurrently, efforts to develop new biomarkers and imaging tests seek to improve screening performance beyond its current limits. This study estimates the mortality reduction, years of life saved, and cost-effectiveness achievable by annual multimodal screening using increasing CA125 to select women for TVS, and predicts improvements achievable by replacing currently available screening tests with hypothetical counterparts with better performance characteristics. An existing stochastic microsimulation model is refined and used to screen a virtual cohort of 1 million women from ages 45 to 85 years. Each woman is assigned a detailed disease course and screening results timeline. The preclinical behavior of CA125 and TVS is simulated using empirical data derived from clinical trials. Simulations in which the disease incidence and performance characteristics of the screening tests are independently varied are conducted to evaluate the impact of these factors on overall screening performance and costs. Our results show that when applied to women at average risk, annual screening using increasing CA125 to select women for TVS achieves modest mortality reduction (~13%) and meets currently accepted cost-effectiveness guidelines. Screening outcomes are relatively insensitive to second-line test performance and costs. Identification of a first-line test that does substantially better than CA125 and has similar costs is required for screening to reduce ovarian mortality by at least 25% and be reasonably cost-effective.
Conflict of interest statement
None of the authors listed above (Charles Drescher, Sarah Hawley, Jason Thorpe, Simone Marticke, Sanjiv Gambhir, Martin McIntosh, and Nicole Urban) has declared any conflict of interest with the above manuscript.
Figures


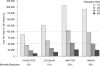
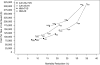
References
-
- Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011 Jun 8;305(22):2295–303. - PubMed
-
- Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009 Apr;10(4):327–40. - PubMed
-
- Skates S, Troiano R, Knapp RC. Longitudinal CA125 detection of sporadic papillary serous carcinoma of the peritoneum. Int J Gynecol Cancer. 2003 Sep-Oct;13(5):693–6. - PubMed
-
- Lu KH, Skates S, Bevers TB, Newland W, Moore RG, Leeds L, et al. A prospective U.S. ovarian cancer screening study using the risk of ovarian cancer algorithm (ROCA) 2010 ASCO Annual Meeting J Clin Oncol. 2010 p. (suppl; abstr 5003)
-
- Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008 Sep;110(3):374–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

