Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression
- PMID: 22751107
- PMCID: PMC3408737
- DOI: 10.1172/JCI61884
Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression
Abstract
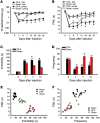
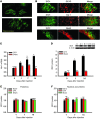
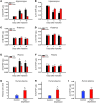
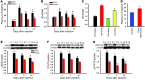
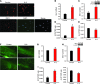
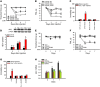
Pain and depression are frequently comorbid disorders, but the mechanism underlying this association is unknown. Here, we report that brain indoleamine 2,3-dioxygenase 1 (IDO1), a rate-limiting enzyme in tryptophan metabolism, plays a key role in this comorbidity. We found that chronic pain in rats induced depressive behavior and IDO1 upregulation in the bilateral hippocampus. Upregulation of IDO1 resulted in the increased kynurenine/tryptophan ratio and decreased serotonin/tryptophan ratio in the bilateral hippocampus. We observed elevated plasma IDO activity in patients with both pain and depression, as well as in rats with anhedonia induced by chronic social stress. Intra-hippocampal administration of IL-6 in rats, in addition to in vitro experiments, demonstrated that IL-6 induces IDO1 expression through the JAK/STAT pathway. Further, either Ido1 gene knockout or pharmacological inhibition of hippocampal IDO1 activity attenuated both nociceptive and depressive behavior. These results reveal an IDO1-mediated regulatory mechanism underlying the comorbidity of pain and depression and suggest a new strategy for the concurrent treatment of both conditions via modulation of brain IDO1 activity.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

