Hooked! Modeling human disease in zebrafish
- PMID: 22751109
- PMCID: PMC3386812
- DOI: 10.1172/JCI60434
Hooked! Modeling human disease in zebrafish
Abstract
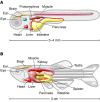
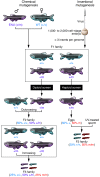
Zebrafish have been widely used as a model system for studying developmental processes, but in the last decade, they have also emerged as a valuable system for modeling human disease. The development and function of zebrafish organs are strikingly similar to those of humans, and the ease of creating mutant or transgenic fish has facilitated the generation of disease models. Here, we highlight the use of zebrafish for defining disease pathways and for discovering new therapies.
Figures

References
-
- Haffter P, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

