Truncated p110 ERBB2 induces mammary epithelial cell migration, invasion and orthotopic xenograft formation, and is associated with loss of phosphorylated STAT5
- PMID: 22751112
- PMCID: PMC3655379
- DOI: 10.1038/onc.2012.256
Truncated p110 ERBB2 induces mammary epithelial cell migration, invasion and orthotopic xenograft formation, and is associated with loss of phosphorylated STAT5
Abstract
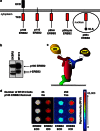
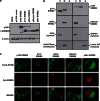
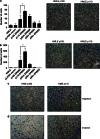
Truncated-ERBB2 isoforms (t-ERBB2s), resulting from receptor proteolysis or alternative translation of the ERBB2 mRNA, exist in a subset of human breast tumors. t-ERBB2s lack the receptor extracellular domain targeted by therapeutic anti-ERBB2 antibodies and antibody-drug conjugates, including trastuzumab, trastuzumab-DM1 and pertuzumab. In clinical studies, expression of t-ERBB2 in breast tumors correlates with metastasis as well as trastuzumab resistance. By using a novel immuno-microarray method, we detect a significant t-ERBB2 fraction in 18 of 31 (58%) of immunohistochemistry (IHC)3+ ERBB2+ human tumor specimens, and further show that t-ERBB2 isoforms are phosphorylated in a subset of IHC3+ samples (10 of 31, 32%). We investigated t-ERBB2 biological activity via engineered expression of full-length and truncated ERBB2 isoforms in human mammary epithelial cells (HMECs), including HMEC and MCF10A cells. Expression of p110 t-ERBB2, but not p95m (m=membrane, also 648CTF) or intracellular ERBB2s, significantly enhanced cell migration and invasion in multiple cell types. In addition, only expression of the p110 isoform led to human breast epithelial cell (HMLE) xenograft formation in vivo. Expression of t-ERBB2s did not result in hyperactivation of the phosphoinositide kinase-3/AKT or mitogen-activated protein kinase signaling pathways in these cells; rather, phosphoproteomic array profiling revealed attenuation of phosphorylated signal transducer and activator of transcription 5 (STAT5) in p110-t-ERBB2-expressing cells compared to controls. Short hairpin-mediated silencing of STAT5 phenocopied p110-t-ERBB2-driven cell migration and invasion, while expression of constitutively active STAT5 reversed these effects. Thus, we provide novel evidence that (1) expression of p110 t-ERBB2 is sufficient for full transformation of HMEC, yielding in vivo xenograft formation, and (2) truncated p110 t-ERBB2 expression is associated with decreased phosphorylation of STAT5.
Figures




Similar articles
-
A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells.Oncogene. 2010 Oct 28;29(43):5839-49. doi: 10.1038/onc.2010.318. Epub 2010 Aug 16. Oncogene. 2010. PMID: 20711231 Free PMC article.
-
Constitutive activation of signal transducer and activator of transcription 5 contributes to tumor growth, epithelial-mesenchymal transition, and resistance to epidermal growth factor receptor targeting.Clin Cancer Res. 2008 Dec 1;14(23):7682-90. doi: 10.1158/1078-0432.CCR-08-1328. Clin Cancer Res. 2008. PMID: 19047094 Free PMC article.
-
Constitutive activation of JAK2 in mammary epithelium elevates Stat5 signalling, promotes alveologenesis and resistance to cell death, and contributes to tumourigenesis.Cell Death Differ. 2012 Mar;19(3):511-22. doi: 10.1038/cdd.2011.122. Epub 2011 Sep 23. Cell Death Differ. 2012. PMID: 21941370 Free PMC article.
-
Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression.J Mammary Gland Biol Neoplasia. 2008 Mar;13(1):93-103. doi: 10.1007/s10911-008-9062-z. Epub 2008 Jan 29. J Mammary Gland Biol Neoplasia. 2008. PMID: 18228120 Review.
-
Crosstalk between STAT5 activation and PI3K/AKT functions in normal and transformed mammary epithelial cells.Mol Cell Endocrinol. 2017 Aug 15;451:31-39. doi: 10.1016/j.mce.2017.04.025. Epub 2017 May 8. Mol Cell Endocrinol. 2017. PMID: 28495456 Free PMC article. Review.
Cited by
-
Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases.Neuro Oncol. 2015 Sep;17(9):1241-9. doi: 10.1093/neuonc/nov012. Epub 2015 Feb 13. Neuro Oncol. 2015. PMID: 25681308 Free PMC article.
-
Label-Free Quantitation and Mapping of the ErbB2 Tumor Receptor by Multiple Protease Digestion with Data-Dependent (MS1) and Data-Independent (MS2) Acquisitions.Int J Proteomics. 2013;2013:791985. doi: 10.1155/2013/791985. Epub 2013 Apr 4. Int J Proteomics. 2013. PMID: 23710360 Free PMC article.
-
Separation-encoded microparticles for single-cell western blotting.Lab Chip. 2020 Jan 7;20(1):64-73. doi: 10.1039/c9lc00917e. Epub 2019 Nov 27. Lab Chip. 2020. PMID: 31773114 Free PMC article.
-
Profiling protein expression in circulating tumour cells using microfluidic western blotting.Nat Commun. 2017 Mar 23;8:14622. doi: 10.1038/ncomms14622. Nat Commun. 2017. PMID: 28332571 Free PMC article.
-
High p95HER2/HER2 Ratio Associated With Poor Outcome in Trastuzumab-Treated HER2-Positive Metastatic Breast Cancer NCCTG N0337 and NCCTG 98-32-52 (Alliance).Clin Cancer Res. 2018 Jul 1;24(13):3053-3058. doi: 10.1158/1078-0432.CCR-17-1864. Epub 2018 Mar 12. Clin Cancer Res. 2018. PMID: 29530935 Free PMC article.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–428. - PubMed
-
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

