Copper-induced intracellular calcium release requires extracellular calcium entry and activation of L-type voltage-dependent calcium channels in Ulva compressa
- PMID: 22751323
- PMCID: PMC3583951
- DOI: 10.4161/psb.20355
Copper-induced intracellular calcium release requires extracellular calcium entry and activation of L-type voltage-dependent calcium channels in Ulva compressa
Abstract
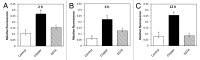
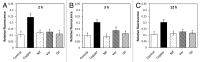
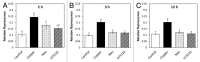
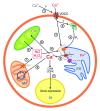
The marine alga Ulva compressa exposed to 10 µM copper showed a triphasic increase of intracellular calcium with maximal levels at 2, 3 and 12 h involving the activation of ryanodine-, Ins(1,4,5)P3- and NAADP-sensitive calcium channels. In order to analyze the requirement of extracellular calcium entry for intracellular calcium release as well as the activation of voltage-dependent calcium channels (VDCC) and phospholipase C, U. compressa was treated with EGTA, a non-permeable calcium chelating agent, with verapamil, nipfedipine and diltiazem, inhibitors of L-type VDCC, and with neomycin and U731222, inhibitors of phospholipase C. The release of intracellular calcium was partially inhibited with EGTA at 2 and 3 h and completely inhibited at 12 h of copper exposure and decreased with inhibitors of L-type VDCC and phospholipase C. Thus, copper-induced intracellular calcium release depends on calcium entry and activation of L-type VDCC and phospholipase C. An integrative model of copper-induced cellular responses in U. compressa is presented.
Figures
Similar articles
-
Copper-induced activation of TRP channels promotes extracellular calcium entry, activation of CaMs and CDPKs, copper entry and membrane depolarization in Ulva compressa.Front Plant Sci. 2015 Mar 20;6:182. doi: 10.3389/fpls.2015.00182. eCollection 2015. Front Plant Sci. 2015. PMID: 25852728 Free PMC article.
-
Copper-induced calcium release from ER involves the activation of ryanodine-sensitive and IP(3)-sensitive channels in Ulva compressa.Plant Signal Behav. 2010 Dec;5(12):1647-9. doi: 10.4161/psb.5.12.13977. Epub 2010 Dec 1. Plant Signal Behav. 2010. PMID: 21139437 Free PMC article.
-
Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess.Plant Physiol. 2012 Mar;158(3):1451-62. doi: 10.1104/pp.111.191759. Epub 2012 Jan 10. Plant Physiol. 2012. PMID: 22234999 Free PMC article.
-
Mechanisms of Copper Tolerance, Accumulation, and Detoxification in the Marine Macroalga Ulva compressa (Chlorophyta): 20 Years of Research.Plants (Basel). 2020 May 27;9(6):681. doi: 10.3390/plants9060681. Plants (Basel). 2020. PMID: 32471287 Free PMC article. Review.
-
Impaired control of L-type voltage-dependent calcium channels in experimental hypertension.Physiol Res. 2009;58 Suppl 2:S43-S54. doi: 10.33549/physiolres.931914. Physiol Res. 2009. PMID: 20131936 Review.
Cited by
-
The Genome of the Marine Alga Ulva compressa (Chlorophyta) Reveals Protein-Coding Genes with Similarity to Plants and Green Microalgae, but Also to Animal, Bacterial, and Fungal Genes.Int J Mol Sci. 2022 Jun 30;23(13):7279. doi: 10.3390/ijms23137279. Int J Mol Sci. 2022. PMID: 35806287 Free PMC article.
-
Calcium signaling-mediated transcriptional reprogramming during abiotic stress response in plants.Theor Appl Genet. 2023 Sep 20;136(10):210. doi: 10.1007/s00122-023-04455-2. Theor Appl Genet. 2023. PMID: 37728763 Review.
-
Submicromolar copper (II) ions stimulate transretinal signaling in the isolated retina from wild type but not from Cav2.3-deficient mice.BMC Ophthalmol. 2020 May 6;20(1):182. doi: 10.1186/s12886-020-01451-8. BMC Ophthalmol. 2020. PMID: 32375703 Free PMC article.
-
Copper-induced activation of TRPs and VDCCs triggers a calcium signature response regulating gene expression in Ectocarpus siliculosus.PeerJ. 2018 Apr 16;6:e4556. doi: 10.7717/peerj.4556. eCollection 2018. PeerJ. 2018. PMID: 29682409 Free PMC article.
-
Copper-induced activation of TRP channels promotes extracellular calcium entry, activation of CaMs and CDPKs, copper entry and membrane depolarization in Ulva compressa.Front Plant Sci. 2015 Mar 20;6:182. doi: 10.3389/fpls.2015.00182. eCollection 2015. Front Plant Sci. 2015. PMID: 25852728 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
