Combination of gastrin-releasing peptide antagonist with cytotoxic agents produces synergistic inhibition of growth of human experimental colon cancers
- PMID: 22751419
- PMCID: PMC3404878
- DOI: 10.4161/cc.20900
Combination of gastrin-releasing peptide antagonist with cytotoxic agents produces synergistic inhibition of growth of human experimental colon cancers
Abstract
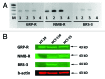
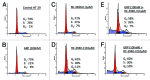
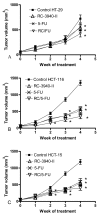
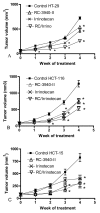
We investigated the efficacy of a powerful antagonist of bombesin/gastrin-releasing peptide (BN/GRP) RC-3940-II administered as a single agent or in combination with cytotoxic agents on the growth of HT-29, HCT-116 and HCT-15 human colon cancer in vitro and in vivo. GRP-receptor mRNA and protein were found in all three cell lines tested. Exposure of HT-29 cells to 10 μM RC-3940-II led to an increase in the number of cells blocked in S phase and G 2/M and cells with lower G(0)/G(1) DNA content. Similar changes on the cell cycle traverse of HT-29 cells could also be seen at lower concentrations of RC-3940-II (1 μM) after pretreatment with 100 nM GRP (14-27), indicating a dose-dependent mechanism of action based on the blockage of BN/GRP induced proliferation of tumor cells at lower concentrations. Daily in vivo treatment with BN/GRP antagonist RC-3940-II decreased the volume of HT-29, HCT-116 and HCT-15 tumors xenografted into athymic nude mice by 25 to 67% (p < 0.005). Combined treatment with RC-3940-II and chemotherapeutic agents 5-FU and irinotecan resulted in a synergistic tumor growth suppression of HT-29, HCT-116 and HCT-15 xenografts by 43% to 78%. In HT-29 and HCT-116 xenografts the inhibition for the combinations of RC-3940-II and irinotecan vs. single substances (p < 0.05) was significantly greater. These findings support the use of RC-3940-II as an anticancer agent and may help to design clinical trials using RC-3940-II in combinations with cytotoxic agents.
Figures
Similar articles
-
Inhibition of growth of MDA-MB-468 estrogen-independent human breast carcinoma by bombesin/gastrin-releasing peptide antagonists RC-3095 and RC-3940-II.Cancer. 2000 Mar 15;88(6):1384-92. doi: 10.1002/(sici)1097-0142(20000315)88:6<1384::aid-cncr16>3.0.co;2-q. Cancer. 2000. PMID: 10717621
-
GHRH antagonist when combined with cytotoxic agents induces S-phase arrest and additive growth inhibition of human colon cancer.Cell Cycle. 2012 Nov 15;11(22):4203-10. doi: 10.4161/cc.22498. Epub 2012 Oct 24. Cell Cycle. 2012. PMID: 23095641 Free PMC article.
-
Powerful inhibition of in-vivo growth of experimental hepatic cancers by bombesin/gastrin-releasing peptide antagonist RC-3940-II.Anticancer Drugs. 2012 Oct;23(9):906-13. doi: 10.1097/CAD.0b013e328354bd25. Anticancer Drugs. 2012. PMID: 22926257
-
Gastrin-releasing peptide as a molecular target for inflammatory diseases: an update.Inflamm Allergy Drug Targets. 2013 Jun;12(3):172-7. doi: 10.2174/1871528111312030003. Inflamm Allergy Drug Targets. 2013. PMID: 23621446 Review.
-
Gastrin-Releasing Peptide Receptor Targeting in Cancer Treatment: Emerging Signaling Networks and Therapeutic Applications.Curr Drug Targets. 2016;17(5):508-14. doi: 10.2174/1389450116666151001112130. Curr Drug Targets. 2016. PMID: 26424393 Review.
Cited by
-
Antagonists of growth hormone-releasing hormone suppress in vivo tumor growth and gene expression in triple negative breast cancers.Oncotarget. 2012 Sep;3(9):988-97. doi: 10.18632/oncotarget.634. Oncotarget. 2012. PMID: 22941871 Free PMC article.
-
HYNIC and DOMA conjugated radiolabeled bombesin analogs as receptor-targeted probes for scintigraphic detection of breast tumor.EJNMMI Res. 2019 Mar 18;9(1):25. doi: 10.1186/s13550-019-0493-x. EJNMMI Res. 2019. PMID: 30887136 Free PMC article.
-
Potentiating effects of GHRH analogs on the response to chemotherapy.Cell Cycle. 2015;14(5):699-704. doi: 10.1080/15384101.2015.1010893. Cell Cycle. 2015. PMID: 25648497 Free PMC article.
-
Suppression of the proliferation of human U-87 MG glioblastoma cells by new antagonists of growth hormone-releasing hormone in vivo and in vitro.Target Oncol. 2013 Dec;8(4):281-90. doi: 10.1007/s11523-013-0264-y. Epub 2013 Feb 1. Target Oncol. 2013. PMID: 23371031
-
Exploiting cancer's phenotypic guise against itself: targeting ectopically expressed peptide G-protein coupled receptors for lung cancer therapy.Oncotarget. 2017 Jun 7;8(61):104615-104637. doi: 10.18632/oncotarget.18403. eCollection 2017 Nov 28. Oncotarget. 2017. PMID: 29262666 Free PMC article. Review.
References
-
- Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–86. doi: 10.1200/JCO.2007.11.3357. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
