Palmitoylation regulates vesicular trafficking of R-Ras to membrane ruffles and effects on ruffling and cell spreading
- PMID: 22751447
- PMCID: PMC3442799
- DOI: 10.4161/sgtp.21084
Palmitoylation regulates vesicular trafficking of R-Ras to membrane ruffles and effects on ruffling and cell spreading
Abstract
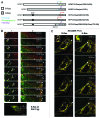
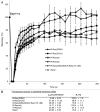
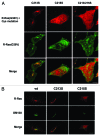
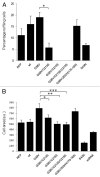
In this study we investigated the dynamics of R-Ras intracellular trafficking and its contributions to the unique roles of R-Ras in membrane ruffling and cell spreading. Wild type and constitutively active R-Ras localized to membranes of both Rab11- and transferrin-positive and -negative vesicles, which trafficked anterograde to the leading edge in migrating cells. H-Ras also co-localized with R-Ras in many of these vesicles in the vicinity of the Golgi, but R-Ras and H-Ras vesicles segregated proximal to the leading edge, in a manner dictated by the C-terminal membrane-targeting sequences. These segregated vesicle trafficking patterns corresponded to distinct modes of targeting to membrane ruffles at the leading edge. Geranylgeranylation was required for membrane anchorage of R-Ras, whereas palmitoylation was required for exit from the Golgi in post-Golgi vesicle membranes and trafficking to the plasma membrane. R-Ras vesicle membranes did not contain phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P(3)), whereas R-Ras co-localized with PtdIns(3,4,5)P(3) in membrane ruffles. Finally, palmitoylation-deficient R-Ras blocked membrane ruffling, R-Ras/PI3-kinase interaction, enrichment of PtdIns(3,4,5)P(3) at the plasma membrane, and R-Ras-dependent cell spreading. Thus, lipid modification of R-Ras dictates its vesicle trafficking, targeting to membrane ruffles, and its unique roles in localizing PtdIns(3,4,5)P(3) to ruffles and promoting cell spreading.
Figures



Similar articles
-
R-Ras regulates beta1-integrin trafficking via effects on membrane ruffling and endocytosis.BMC Cell Biol. 2010 Feb 18;11:14. doi: 10.1186/1471-2121-11-14. BMC Cell Biol. 2010. PMID: 20167113 Free PMC article.
-
Palmitoylation and the trafficking of peripheral membrane proteins.Biochem Soc Trans. 2013 Feb 1;41(1):62-6. doi: 10.1042/BST20120243. Biochem Soc Trans. 2013. PMID: 23356259
-
Legionella-Containing Vacuoles Capture PtdIns(4)P-Rich Vesicles Derived from the Golgi Apparatus.mBio. 2018 Dec 11;9(6):e02420-18. doi: 10.1128/mBio.02420-18. mBio. 2018. PMID: 30538188 Free PMC article.
-
GOLPH3: a Golgi phosphatidylinositol(4)phosphate effector that directs vesicle trafficking and drives cancer.J Lipid Res. 2019 Feb;60(2):269-275. doi: 10.1194/jlr.R088328. Epub 2018 Sep 28. J Lipid Res. 2019. PMID: 30266835 Free PMC article. Review.
-
Palmitoylation cycles and regulation of protein function (Review).Mol Membr Biol. 2009 Jan;26(1):42-54. doi: 10.1080/09687680802680108. Epub 2009 Jan 30. Mol Membr Biol. 2009. PMID: 19169934 Review.
Cited by
-
RLIP76 regulates HIF-1 activity, VEGF expression and secretion in tumor cells, and secretome transactivation of endothelial cells.FASEB J. 2014 Sep;28(9):4158-68. doi: 10.1096/fj.14-255711. Epub 2014 Jun 13. FASEB J. 2014. PMID: 24928198 Free PMC article.
-
An increase in tolerogenic dendritic cell and natural regulatory T cell numbers during experimental autoimmune encephalomyelitis in Rras-/- mice results in attenuated disease.J Immunol. 2014 Jun 1;192(11):5109-17. doi: 10.4049/jimmunol.1302254. Epub 2014 Apr 25. J Immunol. 2014. PMID: 24771856 Free PMC article.
-
R-Ras Regulates Murine T Cell Migration and Intercellular Adhesion Molecule-1 Binding.PLoS One. 2015 Dec 28;10(12):e0145218. doi: 10.1371/journal.pone.0145218. eCollection 2015. PLoS One. 2015. PMID: 26710069 Free PMC article.
-
Plasma growth factors maintain constitutive translation in platelets to regulate reactivity and thrombotic potential.Blood Adv. 2024 Mar 26;8(6):1550-1566. doi: 10.1182/bloodadvances.2023011734. Blood Adv. 2024. PMID: 38163324 Free PMC article.
-
RLIP76 regulates Arf6-dependent cell spreading and migration by linking ARNO with activated R-Ras at recycling endosomes.Biochem Biophys Res Commun. 2015 Nov 27;467(4):785-91. doi: 10.1016/j.bbrc.2015.10.064. Epub 2015 Oct 20. Biochem Biophys Res Commun. 2015. PMID: 26498519 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
