Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer
- PMID: 22751464
- PMCID: PMC3899801
- DOI: 10.1158/0008-5472.CAN-12-0636
Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer
Abstract
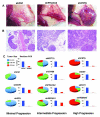
Molecular mechanisms mediating the progression of ductal carcinoma in situ (DCIS) to invasive breast cancer remain largely unknown. We used gene expression profiling of human DCIS (n = 53) and invasive breast cancer (n = 51) to discover uniquely expressed genes that may also regulate progression. There were 470 total differentially expressed genes (≥2-fold; P < 0.05). Elevated expression of genes involved in synthesis and organization of extracellular matrix was particularly prominent in the epithelium of invasive breast cancer. The degree of overlap of the genes with nine similar studies in the literature was determined to help prioritize their potential importance, resulting in 74 showing overlap in ≥2 studies (average 3.6 studies/gene; range 2-8 studies). Using hierarchical clustering, the 74-gene profile correctly categorized 96% of samples in this study and 94% of samples from 3 similar independent studies. To study the progression of DCIS to invasive breast cancer in vivo, we introduced human DCIS cell lines engineered to express specific genes into a "mammary intraductal DCIS" xenograft model. Progression of xenografts to invasive breast cancer was dramatically increased by suppressing four genes that were usually elevated in clinical samples of DCIS, including a protease inhibitor (CSTA) and genes involved in cell adhesion and signaling (FAT1, DST, and TMEM45A), strongly suggesting that they normally function to suppress progression. In summary, we have identified unique gene expression profiles of human DCIS and invasive breast cancer, which include novel genes regulating tumor progression. Targeting some of these genes may improve the detection, diagnosis, and therapy of DCIS.
©2012 AACR.
Figures




References
-
- Allred DC. Biological features of human premalignant breast disease and the progression to cancer. In: Harris JR, Lippman ME, Mlorrow M, Hellman S, Osborne CK, editors. Diseases of the Breast. 4th ed Lippincott Williams and Wilkins; Philadelphia: 2009. pp. 323–34.
-
- Allred DC, Wu Y, Mao S, et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin Cancer Res. 2008;14:370–8. - PubMed
-
- Society AC. Breast Cancer Facts and Figures 2011-2012. 2010.
-
- Kuerer HM, Albarracin CT, Yang WT, et al. Ductal carcinoma in situ: state of the science and roadmap to advance the field. J Clin Oncol. 2009;27:279–88. - PubMed
-
- Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA. Continued local recurrence of carcinoma 15-25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer. 1995;76:1197–200. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

