Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring
- PMID: 22751544
- PMCID: PMC3421881
- DOI: 10.1128/AAC.00626-12
Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring
Abstract
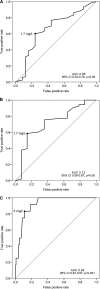
Voriconazole is a first-line agent in the treatment of many invasive fungal infections and is known to display highly variable pharmacokinetics. Previous studies of voriconazole therapeutic drug monitoring (TDM) have suggested concentration monitoring to be clinically useful but have been limited by small patient samples at a single institution. This multicenter retrospective study aimed to investigate relationships between voriconazole concentration and clinical outcomes and adverse events and to assess clinical factors and drug interactions that may affect voriconazole concentration. Medical records were reviewed for patients who received voriconazole and had at least 1 concentration measured at seven hospitals in Australia. The study included 201 patients with 783 voriconazole trough concentrations. Voriconazole concentrations of <1.7 mg/liter were associated with a significantly greater incidence of treatment failure (19/74 patients [26%]) than concentrations of ≥1.7 mg/liter (6/89 patients [7%]) (P < 0.01). Neurotoxic adverse events (visual and auditory hallucinations) occurred more frequently at voriconazole concentrations of >5 mg/liter (10/31 patients [32%]) than at concentrations of ≤5 mg/liter (2/170 patients [1.2%]) (P < 0.01). Multiple regression analysis of voriconazole concentration identified associations between increasing patient weight, oral administration of voriconazole, and coadministration of phenytoin or rifampin and significantly reduced concentrations, and associations between increasing patient age and coadministration of proton pump inhibitors and increased concentrations. Coadministration of glucocorticoids was found to significantly reduce voriconazole concentrations, inferring a previously unreported drug interaction between glucocorticoids and voriconazole.
Figures

Similar articles
-
Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration.Antimicrob Agents Chemother. 2012 Nov;56(11):5503-10. doi: 10.1128/AAC.00802-12. Epub 2012 Aug 13. Antimicrob Agents Chemother. 2012. PMID: 22890761 Free PMC article.
-
Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity.Clin Chim Acta. 2013 Sep 23;424:119-22. doi: 10.1016/j.cca.2013.05.025. Epub 2013 Jun 6. Clin Chim Acta. 2013. PMID: 23747486
-
Voriconazole concentrations and outcome of invasive fungal infections.Clin Microbiol Infect. 2010 Jul;16(7):927-33. doi: 10.1111/j.1469-0691.2009.02990.x. Epub 2009 Oct 20. Clin Microbiol Infect. 2010. PMID: 19845698
-
Clinical application of voriconazole concentrations in the treatment of invasive aspergillosis.Ann Pharmacother. 2008 Dec;42(12):1859-64. doi: 10.1345/aph.1L243. Epub 2008 Nov 18. Ann Pharmacother. 2008. PMID: 19017830 Review.
-
Pharmacology and clinical use of voriconazole.Expert Opin Drug Metab Toxicol. 2010 Jan;6(1):83-94. doi: 10.1517/17425250903463878. Expert Opin Drug Metab Toxicol. 2010. PMID: 19947892 Review.
Cited by
-
Microdialysis of Voriconazole and its N-Oxide Metabolite: Amalgamating Knowledge of Distribution and Metabolism Processes in Humans.Pharm Res. 2022 Dec;39(12):3279-3291. doi: 10.1007/s11095-022-03407-7. Epub 2022 Oct 21. Pharm Res. 2022. PMID: 36271205 Free PMC article. Clinical Trial.
-
Associated factors with voriconazole plasma concentration: a systematic review and meta-analysis.Front Pharmacol. 2024 Aug 23;15:1368274. doi: 10.3389/fphar.2024.1368274. eCollection 2024. Front Pharmacol. 2024. PMID: 39246651 Free PMC article.
-
Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America.Clin Infect Dis. 2016 Aug 15;63(4):e1-e60. doi: 10.1093/cid/ciw326. Epub 2016 Jun 29. Clin Infect Dis. 2016. PMID: 27365388 Free PMC article.
-
Voriconazole Pharmacokinetics in Critically Ill Patients and Extracorporeal Membrane Oxygenation Support: A Retrospective Comparative Case-Control Study.Antibiotics (Basel). 2023 Jun 25;12(7):1100. doi: 10.3390/antibiotics12071100. Antibiotics (Basel). 2023. PMID: 37508196 Free PMC article.
-
How to manage aspergillosis in non-neutropenic intensive care unit patients.Crit Care. 2014 Jul 25;18(4):458. doi: 10.1186/s13054-014-0458-4. Crit Care. 2014. PMID: 25167934 Free PMC article. Review.
References
-
- Blume H, Donath F, Warnke A, Schug BS. 2006. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 29:769–784 - PubMed
-
- Boyd AE, et al. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241–1244 - PubMed
-
- Chen Y, Ferguson SS, Negishi M, Goldstein JA. 2003. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol. Pharmacol. 64:316–324 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

