Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring
- PMID: 22751544
- PMCID: PMC3421881
- DOI: 10.1128/AAC.00626-12
Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring
Abstract
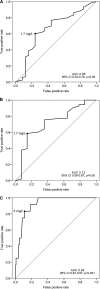
Voriconazole is a first-line agent in the treatment of many invasive fungal infections and is known to display highly variable pharmacokinetics. Previous studies of voriconazole therapeutic drug monitoring (TDM) have suggested concentration monitoring to be clinically useful but have been limited by small patient samples at a single institution. This multicenter retrospective study aimed to investigate relationships between voriconazole concentration and clinical outcomes and adverse events and to assess clinical factors and drug interactions that may affect voriconazole concentration. Medical records were reviewed for patients who received voriconazole and had at least 1 concentration measured at seven hospitals in Australia. The study included 201 patients with 783 voriconazole trough concentrations. Voriconazole concentrations of <1.7 mg/liter were associated with a significantly greater incidence of treatment failure (19/74 patients [26%]) than concentrations of ≥1.7 mg/liter (6/89 patients [7%]) (P < 0.01). Neurotoxic adverse events (visual and auditory hallucinations) occurred more frequently at voriconazole concentrations of >5 mg/liter (10/31 patients [32%]) than at concentrations of ≤5 mg/liter (2/170 patients [1.2%]) (P < 0.01). Multiple regression analysis of voriconazole concentration identified associations between increasing patient weight, oral administration of voriconazole, and coadministration of phenytoin or rifampin and significantly reduced concentrations, and associations between increasing patient age and coadministration of proton pump inhibitors and increased concentrations. Coadministration of glucocorticoids was found to significantly reduce voriconazole concentrations, inferring a previously unreported drug interaction between glucocorticoids and voriconazole.
Figures

References
-
- Blume H, Donath F, Warnke A, Schug BS. 2006. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 29:769–784 - PubMed
-
- Boyd AE, et al. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241–1244 - PubMed
-
- Chen Y, Ferguson SS, Negishi M, Goldstein JA. 2003. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol. Pharmacol. 64:316–324 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

