Enhancing apoptotic cell clearance mitigates bacterial translocation and promotes tissue repair after gut ischemia-reperfusion injury
- PMID: 22751701
- PMCID: PMC3573750
- DOI: 10.3892/ijmm.2012.1044
Enhancing apoptotic cell clearance mitigates bacterial translocation and promotes tissue repair after gut ischemia-reperfusion injury
Abstract
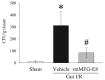
A key aspect of intestinal ischemia/reperfusion (I/R) injury is the increased occurrence of apoptotic cell death in the gut. Insufficient clearance of apoptotic cells leads to increased inflammation and impaired tissue repair. Our recent studies have shown that administration of milk fat globule-epidermal growth factor-factor 8 (MFG-E8), a crucial molecule for apoptotic cell clearance, reduces apoptosis and inflammation under various disease conditions. The purpose of this study was to determine whether MFG-E8 reduces bacterial translocation and promotes tissue repair in a mouse model of gut I/R. Gut ischemia was induced by placing a microvascular clip across the superior mesenteric artery for 90 min in male adult mice. After removing the clip, recombinant murine MFG-E8 (rmMFG-E8) (0.4 µg/20 g BW) or normal saline (Vehicle) was intraperitoneally injected. At 4 h after reperfusion, apoptosis in the gut was measured by TUNEL staining. The mesenteric lymph node (MLN) complex was homogenized and plated on chocolate agar plates for bacterial culture. Neutrophil infiltration was assessed by examining myeloperoxidase (MPO) activity in the gut. Vascular endothelial growth factor (VEGF) levels in the gut, an indicator of tissue repair, were measured by western blotting. Out results showed that TUNEL-positive staining in the gut increased significantly in gut I/R vehicle-treated mice. Treatment with rmMFG-E8 markedly suppressed the number of apoptotic cells. Bacterial translocation to the MLN was minimal in sham mice, but was extensive in gut I/R vehicle-treated mice. rmMFG-E8 treatment significantly reduced bacterial translocation to the MLN. Similarly, gut I/R induced a significant increase in intestinal MPO activities in vehicle-treated mice. rmMFG-E8 treatment markedly reduced the increase in intestinal MPO activities after gut I/R. Intestinal levels of VEGF decreased significantly at 4 h after gut I/R. rmMFG-E8 treatment significantly increased intestinal VEGF levels. Thus, enhancing apoptotic cell clearance by rmMFG-E8 mitigates bacterial translocation, inhibits neutrophil infiltration and promotes tissue repair after gut I/R. Enhancing apoptotic cell clearance can be a novel concept in the treatment of gut I/R injury.
Figures







Similar articles
-
Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion.Am J Respir Crit Care Med. 2010 Feb 1;181(3):238-46. doi: 10.1164/rccm.200804-625OC. Epub 2009 Nov 5. Am J Respir Crit Care Med. 2010. PMID: 19892861 Free PMC article.
-
Milk fat globule-epidermal growth factor-factor VIII attenuates sepsis-induced acute kidney injury.J Surg Res. 2017 Jun 1;213:281-289. doi: 10.1016/j.jss.2017.02.024. Epub 2017 Feb 24. J Surg Res. 2017. PMID: 28601327 Free PMC article.
-
Milk fat globule EGF factor 8 attenuates sepsis-induced apoptosis and organ injury in alcohol-intoxicated rats.Alcohol Clin Exp Res. 2010 Sep 1;34(9):1625-33. doi: 10.1111/j.1530-0277.2010.01248.x. Epub 2010 Jun 25. Alcohol Clin Exp Res. 2010. PMID: 20586751 Free PMC article.
-
Milk fat-globule epidermal growth factor 8: A potential Regulator of Cutaneous Wound Healing.Mol Biol Rep. 2022 Sep;49(9):8883-8893. doi: 10.1007/s11033-022-07365-6. Epub 2022 May 17. Mol Biol Rep. 2022. PMID: 35581508 Review.
-
Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury.Mol Med. 2011 Jan-Feb;17(1-2):126-33. doi: 10.2119/molmed.2010.00135. Epub 2010 Sep 21. Mol Med. 2011. PMID: 20882259 Free PMC article. Review.
Cited by
-
Recombinant human milk fat globule-EGF factor VIII (rhMFG-E8) as a therapy for sepsis after acute exposure to alcohol.Mol Med. 2019 Nov 20;25(1):52. doi: 10.1186/s10020-019-0118-x. Mol Med. 2019. PMID: 31747882 Free PMC article.
-
Protective effect of MFG-E8 after cutaneous ischemia-reperfusion injury.J Invest Dermatol. 2015 Apr;135(4):1157-1165. doi: 10.1038/jid.2014.515. Epub 2014 Dec 10. J Invest Dermatol. 2015. PMID: 25493650
-
Role of milk fat globule-epidermal growth factor 8 in osteoimmunology.Bonekey Rep. 2016 Jul 20;5:820. doi: 10.1038/bonekey.2016.52. eCollection 2016. Bonekey Rep. 2016. PMID: 27579162 Free PMC article. Review.
-
Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research.Am J Physiol Gastrointest Liver Physiol. 2015 Jan 15;308(2):G63-75. doi: 10.1152/ajpgi.00112.2013. Epub 2014 Nov 20. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25414098 Free PMC article. Review.
-
A bibliometric analysis of exosomes in sepsis from 2004 to 2022.Medicine (Baltimore). 2023 Aug 4;102(31):e34613. doi: 10.1097/MD.0000000000034613. Medicine (Baltimore). 2023. PMID: 37543762 Free PMC article. Review.
References
-
- Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054–1062. - PubMed
-
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954–968. - PubMed
-
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

