Loss of the androgen receptor cofactor p44/WDR77 induces astrogliosis
- PMID: 22751923
- PMCID: PMC3421997
- DOI: 10.1128/MCB.00298-12
Loss of the androgen receptor cofactor p44/WDR77 induces astrogliosis
Abstract
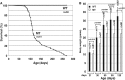
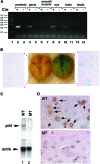
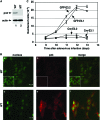
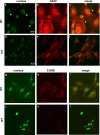
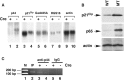
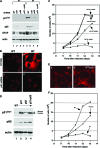
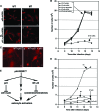
Astrogliosis is induced by neuronal damage and is also a pathological feature of the major aging-related neurodegenerative disorders. The mechanisms that control the cascade of astrogliosis have not been well established. In a previous study, we identified a novel androgen receptor (AR)-interacting protein, p44/WDR77, that plays a critical role in the proliferation and differentiation of prostate epithelial cells. In the present study, we found that deletion of the p44/WDR77 gene caused premature death with dramatic astrogliosis in mouse brain. We further found that p44/WDR77 is expressed in astrocytes and that loss of p44/WDR77 expression in astrocytes leads to growth arrest and astrogliosis. The astrocyte activation induced by deletion of the p44/WDR77 gene was associated with upregulation of p21(Cip1) expression and NF-κB activation. Silencing p21(Cip1) or NF-κB p65 expression with short hairpin RNA (shRNA) abolished astrocyte activation and rescued the astrocyte growth inhibition induced by deletion of the p44/WDR77 gene. Our results reveal a novel role for p44/WDR77 in the control of astrocyte activation through p21(Cip1) and NF-κB signaling.
Figures


Similar articles
-
cGMP-dependent protein kinase Iβ interacts with p44/WDR77 to regulate androgen receptor-driven gene expression.PLoS One. 2013 Jun 3;8(6):e63119. doi: 10.1371/journal.pone.0063119. Print 2014. PLoS One. 2013. PMID: 23755100 Free PMC article.
-
The p44/wdr77-dependent cellular proliferation process during lung development is reactivated in lung cancer.Oncogene. 2013 Apr 11;32(15):1888-900. doi: 10.1038/onc.2012.207. Epub 2012 Jun 4. Oncogene. 2013. PMID: 22665061 Free PMC article.
-
Altered differentiation and proliferation of prostate epithelium in mice lacking the androgen receptor cofactor p44/WDR77.Endocrinology. 2010 Aug;151(8):3941-53. doi: 10.1210/en.2009-1080. Epub 2010 Jun 2. Endocrinology. 2010. PMID: 20519372 Free PMC article.
-
Nuclear transport signals control cellular localization and function of androgen receptor cofactor p44/WDR77.PLoS One. 2011;6(7):e22395. doi: 10.1371/journal.pone.0022395. Epub 2011 Jul 15. PLoS One. 2011. PMID: 21789256 Free PMC article.
-
P44/WDR77 restricts the sensitivity of proliferating cells to TGFβ signaling.Biochem Biophys Res Commun. 2014 Jul 18;450(1):409-15. doi: 10.1016/j.bbrc.2014.05.125. Epub 2014 Jun 2. Biochem Biophys Res Commun. 2014. PMID: 24944016
Cited by
-
The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond.Cell Mol Life Sci. 2015 Jun;72(11):2041-59. doi: 10.1007/s00018-015-1847-9. Epub 2015 Feb 7. Cell Mol Life Sci. 2015. PMID: 25662273 Free PMC article. Review.
-
Signaling pathways regulating neuron-glia interaction and their implications in Alzheimer's disease.J Neurochem. 2016 Feb;136(3):475-91. doi: 10.1111/jnc.13424. Epub 2015 Nov 30. J Neurochem. 2016. PMID: 26546579 Free PMC article. Review.
-
Germ-line mutations in WDR77 predispose to familial papillary thyroid cancer.Proc Natl Acad Sci U S A. 2021 Aug 3;118(31):e2026327118. doi: 10.1073/pnas.2026327118. Proc Natl Acad Sci U S A. 2021. PMID: 34326253 Free PMC article.
References
-
- Altuwaijri S, et al. 2003. Interruption of nuclear factor kappaB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res. 63:7106–7112 - PubMed
-
- Bancroft J. 2005. The endocrinology of sexual arousal. J. Endocrinol. 186:411–427 - PubMed
-
- Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. 2007. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur. J. Neurosci. 25:3039–3046 - PubMed
-
- Besson A, Dowdy SF, Roberts JM. 2008. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell 14:159–169 - PubMed
-
- Coers S, Tanzer L, Jones KJ. 2002. Testosterone treatment attenuates the effects of facial nerve transection on glial fibrillary acidic protein (GFAP) levels in the hamster facial motor nucleus. Metab. Brain Dis. 17:55–63 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
