Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis
- PMID: 22751926
- PMCID: PMC3422013
- DOI: 10.1128/MCB.00125-12
Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis
Abstract
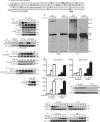
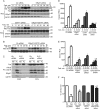
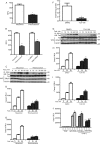
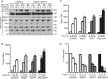
We recently demonstrated that S-glutathionylation of the death receptor Fas (Fas-SSG) amplifies apoptosis (V. Anathy et al., J. Cell Biol. 184:241-252, 2009). In the present study, we demonstrate that distinct pools of Fas exist in cells. Upon ligation of surface Fas, a separate pool of latent Fas in the endoplasmic reticulum (ER) underwent rapid oxidative processing characterized by the loss of free sulfhydryl content (Fas-SH) and resultant increases in S-glutathionylation of Cys294, leading to increases of surface Fas. Stimulation with FasL rapidly induced associations of Fas with ERp57 and glutathione S-transferase π (GSTP), a protein disulfide isomerase and catalyst of S-glutathionylation, respectively, in the ER. Knockdown or inhibition of ERp57 and GSTP1 substantially decreased FasL-induced oxidative processing and S-glutathionylation of Fas, resulting in decreased death-inducing signaling complex formation and caspase activity and enhanced survival. Bleomycin-induced pulmonary fibrosis was accompanied by increased interactions between Fas-ERp57-GSTP1 and S-glutathionylation of Fas. Importantly, fibrosis was largely prevented following short interfering RNA-mediated ablation of ERp57 and GSTP. Collectively, these findings illuminate a regulatory switch, a ligand-initiated oxidative processing of latent Fas, that controls the strength of apoptosis.
Figures




Similar articles
-
Glutaredoxin-1 attenuates S-glutathionylation of the death receptor fas and decreases resolution of Pseudomonas aeruginosa pneumonia.Am J Respir Crit Care Med. 2014 Feb 15;189(4):463-74. doi: 10.1164/rccm.201310-1905OC. Am J Respir Crit Care Med. 2014. PMID: 24325366 Free PMC article.
-
Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas.J Cell Biol. 2009 Jan 26;184(2):241-52. doi: 10.1083/jcb.200807019. J Cell Biol. 2009. PMID: 19171757 Free PMC article.
-
Glutathione S-Transferase P-Mediated Protein S-Glutathionylation of Resident Endoplasmic Reticulum Proteins Influences Sensitivity to Drug-Induced Unfolded Protein Response.Antioxid Redox Signal. 2017 Feb 20;26(6):247-261. doi: 10.1089/ars.2015.6486. Epub 2016 Mar 16. Antioxid Redox Signal. 2017. PMID: 26838680 Free PMC article.
-
S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response.Mol Interv. 2007 Dec;7(6):313-24. doi: 10.1124/mi.7.6.7. Mol Interv. 2007. PMID: 18199853 Free PMC article. Review.
-
The role of the Fas/FasL signaling pathway in environmental toxicant-induced testicular cell apoptosis: An update.Syst Biol Reprod Med. 2018 Apr;64(2):93-102. doi: 10.1080/19396368.2017.1422046. Epub 2018 Jan 4. Syst Biol Reprod Med. 2018. PMID: 29299971 Review.
Cited by
-
Oxidative stress and pulmonary fibrosis.Biochim Biophys Acta. 2013 Jul;1832(7):1028-40. doi: 10.1016/j.bbadis.2012.11.021. Epub 2012 Dec 5. Biochim Biophys Acta. 2013. PMID: 23219955 Free PMC article. Review.
-
Glutaredoxin-1 attenuates S-glutathionylation of the death receptor fas and decreases resolution of Pseudomonas aeruginosa pneumonia.Am J Respir Crit Care Med. 2014 Feb 15;189(4):463-74. doi: 10.1164/rccm.201310-1905OC. Am J Respir Crit Care Med. 2014. PMID: 24325366 Free PMC article.
-
Glutaredoxin-1 modulates the NF-κB signaling pathway to activate inducible nitric oxide synthase in experimental necrotizing enterocolitis.Mol Ther Methods Clin Dev. 2024 Feb 19;32(1):101214. doi: 10.1016/j.omtm.2024.101214. eCollection 2024 Mar 14. Mol Ther Methods Clin Dev. 2024. PMID: 38496303 Free PMC article.
-
Enolase 1 (ENO1) and protein disulfide-isomerase associated 3 (PDIA3) regulate Wnt/β-catenin-driven trans-differentiation of murine alveolar epithelial cells.Dis Model Mech. 2015 Aug 1;8(8):877-90. doi: 10.1242/dmm.019117. Epub 2015 May 14. Dis Model Mech. 2015. PMID: 26035385 Free PMC article.
-
Peroxiredoxins and Beyond; Redox Systems Regulating Lung Physiology and Disease.Antioxid Redox Signal. 2019 Nov 10;31(14):1070-1091. doi: 10.1089/ars.2019.7752. Epub 2019 Apr 5. Antioxid Redox Signal. 2019. PMID: 30799628 Free PMC article. Review.
References
-
- Aoshiba K, Yasui S, Tamaoki J, Nagai A. 2000. The Fas/Fas-ligand system is not required for bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 162: 695– 700 - PubMed
-
- Appenzeller-Herzog C, Ellgaard L. 2008. The human PDI family: versatility packed into a single fold. Biochim. Biophys. Acta 1783: 535– 548 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA085660/CA/NCI NIH HHS/United States
- R01 HL085464/HL/NHLBI NIH HHS/United States
- S10 RR019246/RR/NCRR NIH HHS/United States
- HL085464/HL/NHLBI NIH HHS/United States
- P30 RR031158/RR/NCRR NIH HHS/United States
- R01 HL079331/HL/NHLBI NIH HHS/United States
- R01 HL085646/HL/NHLBI NIH HHS/United States
- P30 RR 031158/RR/NCRR NIH HHS/United States
- R01 CA85660/CA/NCI NIH HHS/United States
- R01 ES021476/ES/NIEHS NIH HHS/United States
- HL060014/HL/NHLBI NIH HHS/United States
- R01 HL060014/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
