Regulation of insulin signaling by the phosphatidylinositol 3,4,5-triphosphate phosphatase SKIP through the scaffolding function of Pak1
- PMID: 22751929
- PMCID: PMC3422014
- DOI: 10.1128/MCB.00636-12
Regulation of insulin signaling by the phosphatidylinositol 3,4,5-triphosphate phosphatase SKIP through the scaffolding function of Pak1
Abstract
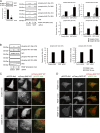
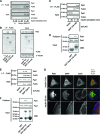
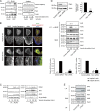
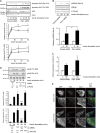
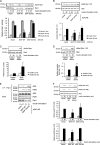
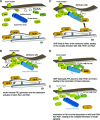
Skeletal muscle and kidney-enriched inositol polyphosphate phosphatase (SKIP) has previously been implicated in the regulation of insulin signaling in skeletal muscle. Here, we present the first report of the mechanisms by which SKIP specifically suppresses insulin signaling and the subsequent glucose uptake. Upon insulin stimulation, SKIP is translocated to the membrane ruffles, where it binds to the active form of Pak1, which mediates multiple protein complex formation with phosphatidylinositol 3,4,5-triphosphate (PIP(3)) effectors such as Akt2, PDK1, and Rac1; this leads to inactivation of these proteins. SKIP also promotes the inhibition of Rac1-dependent kinase activity and the scaffolding function of Pak1, which results in the dissociation of Akt2 and PDK1 from Pak1. Thus, specific suppression of insulin signaling is achieved via the spatiotemporal regulation of SKIP through the scaffolding function of Pak1. These interactions are the foundation of the specific and prominent role of SKIP in the regulation of insulin signaling.
Figures




References
-
- Carpten JD, et al. 2007. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448: 439– 444 - PubMed
-
- Cho H, et al. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728– 1731 - PubMed
-
- Christoforidis S, Zerial M. 2000. Purification and identification of novel Rab effectors using affinity chromatography. Methods 20: 403– 410 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
