Cutaneous structural and biochemical correlates of foot complications in high-risk diabetes
- PMID: 22751961
- PMCID: PMC3424985
- DOI: 10.2337/dc11-2076
Cutaneous structural and biochemical correlates of foot complications in high-risk diabetes
Abstract
Objective: Impairment of skin quality may contribute to diabetic foot ulceration (DFU). Our goal was to determine whether high-risk patients exhibited specific skin structural and metabolic deficits that could predispose to foot complications.
Research design and methods: A total of 46 patients comprising 9 diabetic control subjects, 16 with diabetic peripheral neuropathy (DPN) alone, and 21 with recurrent DFUs (including 9 with Charcot neuroarthropathy [CNA]) were recruited and compared with 14 nondiabetic control (NDC) subjects. DPN was assessed using the Michigan Neuropathy Screening Instrument (MNSI). Skin punch biopsies (3 mm) were performed on upper and lower leg skin for measurements of intraepidermal nerve fiber density (IENFD), structural analysis, type 1 procollagen abundance, tissue degrading matrix metalloproteinases (MMPs), and poly(ADP-ribose) (PAR) immunoreactivity.
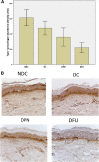
Results: MNSI scores were comparable across DPN groups. IENFD was decreased by diabetes and DPN but did not differ between neuropathic groups. Skin structural deficit scores were elevated in all neuropathic subjects, particularly in the DFU group. Type 1 procollagen abundance was reduced in DFU subjects 387 ± 256 units (mean ± 1 SD) compared with NDC subjects (715 ± 100, P < 0.001). MMP-1 and MMP-2 were activated by diabetes. PAR immunoreactivity was increased in DFU (particularly in the CNA group; P < 0.01) compared with other DPN subjects.
Conclusions: Increased PAR, reduced type 1 procollagen abundance, and impaired skin structure are associated with foot complications in diabetes. The potential of therapies that improve skin quality to reduce DFU needs to be investigated.
Figures
Similar articles
-
The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes.J Diabetes Complications. 2016 Sep-Oct;30(7):1315-20. doi: 10.1016/j.jdiacomp.2016.05.025. Epub 2016 Jun 2. J Diabetes Complications. 2016. PMID: 27324704
-
Effects of a synthetic retinoid on skin structure, matrix metalloproteinases, and procollagen in healthy and high-risk subjects with diabetes.J Diabetes Complications. 2011 Nov-Dec;25(6):398-404. doi: 10.1016/j.jdiacomp.2011.10.002. Epub 2011 Nov 4. J Diabetes Complications. 2011. PMID: 22055260 Free PMC article.
-
Advanced glycation end products assessed by skin autofluorescence: a new marker of diabetic foot ulceration.Diabetes Technol Ther. 2013 Jul;15(7):601-5. doi: 10.1089/dia.2013.0009. Epub 2013 Apr 30. Diabetes Technol Ther. 2013. PMID: 23631605
-
Screening tools for diabetic foot ulcers: a narrative review.Hormones (Athens). 2025 Mar;24(1):71-83. doi: 10.1007/s42000-024-00598-z. Epub 2024 Sep 4. Hormones (Athens). 2025. PMID: 39227550 Review.
-
Impaired dermal microvascular reactivity and implications for diabetic wound formation and healing: an evidence review.J Wound Care. 2020 Sep 1;29(Sup9):S21-S28. doi: 10.12968/jowc.2020.29.Sup9.S21. J Wound Care. 2020. PMID: 32924808 Review.
Cited by
-
The impact of sleep disorders on microvascular complications in patients with type 2 diabetes (SLEEP T2D): the protocol of a cohort study and feasibility randomised control trial.Pilot Feasibility Stud. 2021 Mar 22;7(1):80. doi: 10.1186/s40814-021-00817-z. Pilot Feasibility Stud. 2021. PMID: 33752759 Free PMC article.
-
Skin characterization of diabetes mellitus revealed by polarization-sensitive optical coherence tomography imaging.J Biomed Opt. 2024 Mar;29(3):036003. doi: 10.1117/1.JBO.29.3.036003. Epub 2024 Mar 13. J Biomed Opt. 2024. PMID: 38481479 Free PMC article.
-
Evaluation of Immunomodulatory Responses and Changed Wound Healing in Type 2 Diabetes-A Study Exploiting Dermal Fibroblasts from Diabetic and Non-Diabetic Human Donors.Cells. 2021 Oct 28;10(11):2931. doi: 10.3390/cells10112931. Cells. 2021. PMID: 34831154 Free PMC article.
-
Sleep disturbances and progression of mobility disability: Longitudinal findings from the Nurses' Health Study.Sleep Epidemiol. 2024 Dec;4:100071. doi: 10.1016/j.sleepe.2023.100071. Epub 2023 Dec 14. Sleep Epidemiol. 2024. PMID: 39823032 Free PMC article.
-
Histomorphological and biochemical properties of plantar soft tissue in diabetes.Foot (Edinb). 2017 Dec;33:1-6. doi: 10.1016/j.foot.2017.06.001. Epub 2017 Jun 7. Foot (Edinb). 2017. PMID: 29126035 Free PMC article.
References
-
- Edmonds ME. The diabetic foot. Diabetes Metab Res Rev 2004;20(Suppl. 1):S9–S12 - PubMed
-
- Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice: Neuropathic diabetic foot ulcers. N Engl J Med 2004;351:48–55 - PubMed
-
- Varani J, Warner RL, Phan SH, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000;114:480–486 - PubMed
-
- Virág L, Szabó C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 2002;54:375–429 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

