Epimerase (Msed_0639) and mutase (Msed_0638 and Msed_2055) convert (S)-methylmalonyl-coenzyme A (CoA) to succinyl-CoA in the Metallosphaera sedula 3-hydroxypropionate/4-hydroxybutyrate cycle
- PMID: 22752162
- PMCID: PMC3416614
- DOI: 10.1128/AEM.01312-12
Epimerase (Msed_0639) and mutase (Msed_0638 and Msed_2055) convert (S)-methylmalonyl-coenzyme A (CoA) to succinyl-CoA in the Metallosphaera sedula 3-hydroxypropionate/4-hydroxybutyrate cycle
Abstract
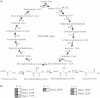
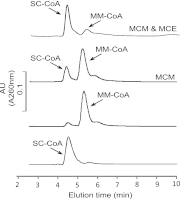
Crenarchaeotal genomes encode the 3-hydroxypropionate/4-hydroxybutyrate (3-HP/4-HB) cycle for carbon dioxide fixation. Of the 13 enzymes putatively comprising the cycle, several of them, including methylmalonyl-coenzyme A (CoA) epimerase (MCE) and methylmalonyl-CoA mutase (MCM), which convert (S)-methylmalonyl-CoA to succinyl-CoA, have not been confirmed and characterized biochemically. In the genome of Metallosphaera sedula (optimal temperature [T(opt)], 73°C), the gene encoding MCE (Msed_0639) is adjacent to that encoding the catalytic subunit of MCM-α (Msed_0638), while the gene for the coenzyme B(12)-binding subunit of MCM (MCM-β) is located remotely (Msed_2055). The expression of all three genes was significantly upregulated under autotrophic compared to heterotrophic growth conditions, implying a role in CO(2) fixation. Recombinant forms of MCE and MCM were produced in Escherichia coli; soluble, active MCM was produced only if MCM-α and MCM-β were coexpressed. MCE is a homodimer and MCM is a heterotetramer (α(2)β(2)) with specific activities of 218 and 2.2 μmol/min/mg, respectively, at 75°C. The heterotetrameric MCM differs from the homo- or heterodimeric orthologs in other organisms. MCE was activated by divalent cations (Ni(2+), Co(2+), and Mg(2+)), and the predicted metal binding/active sites were identified through sequence alignments with less-thermophilic MCEs. The conserved coenzyme B(12)-binding motif (DXHXXG-SXL-GG) was identified in M. sedula MCM-β. The two enzymes together catalyzed the two-step conversion of (S)-methylmalonyl-CoA to succinyl-CoA, consistent with their proposed role in the 3-HP/4-HB cycle. Based on the highly conserved occurrence of single copies of MCE and MCM in Sulfolobaceae genomes, the M. sedula enzymes are likely to be representatives of these enzymes in the 3-HP/4-HB cycle in crenarchaeal thermoacidophiles.
Figures




Similar articles
-
Role of 4-hydroxybutyrate-CoA synthetase in the CO2 fixation cycle in thermoacidophilic archaea.J Biol Chem. 2013 Feb 8;288(6):4012-22. doi: 10.1074/jbc.M112.413195. Epub 2012 Dec 20. J Biol Chem. 2013. PMID: 23258541 Free PMC article.
-
Conversion of 4-hydroxybutyrate to acetyl coenzyme A and its anapleurosis in the Metallosphaera sedula 3-hydroxypropionate/4-hydroxybutyrate carbon fixation pathway.Appl Environ Microbiol. 2014 Apr;80(8):2536-45. doi: 10.1128/AEM.04146-13. Epub 2014 Feb 14. Appl Environ Microbiol. 2014. PMID: 24532060 Free PMC article.
-
Characterization of acetyl-CoA/propionyl-CoA carboxylase in Metallosphaera sedula. Carboxylating enzyme in the 3-hydroxypropionate cycle for autotrophic carbon fixation.Eur J Biochem. 2003 Feb;270(4):736-44. doi: 10.1046/j.1432-1033.2003.03434.x. Eur J Biochem. 2003. PMID: 12581213
-
Controlling the reactivity of radical intermediates by coenzyme B(12)-dependent methylmalonyl-CoA mutase.Biochem Soc Trans. 2002 Aug;30(4):621-4. doi: 10.1042/bst0300621. Biochem Soc Trans. 2002. PMID: 12196149 Review.
-
Role of vitamin B12 on methylmalonyl-CoA mutase activity.J Zhejiang Univ Sci B. 2012 Jun;13(6):423-37. doi: 10.1631/jzus.B1100329. J Zhejiang Univ Sci B. 2012. PMID: 22661206 Free PMC article. Review.
Cited by
-
Reaction kinetic analysis of the 3-hydroxypropionate/4-hydroxybutyrate CO2 fixation cycle in extremely thermoacidophilic archaea.Metab Eng. 2016 Nov;38:446-463. doi: 10.1016/j.ymben.2016.10.009. Epub 2016 Oct 19. Metab Eng. 2016. PMID: 27771364 Free PMC article.
-
Novel Transcriptional Regulons for Autotrophic Cycle Genes in Crenarchaeota.J Bacteriol. 2015 Jul;197(14):2383-91. doi: 10.1128/JB.00249-15. Epub 2015 May 4. J Bacteriol. 2015. PMID: 25939834 Free PMC article.
-
Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide.Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5840-5. doi: 10.1073/pnas.1222607110. Epub 2013 Mar 25. Proc Natl Acad Sci U S A. 2013. PMID: 23530213 Free PMC article.
-
Role of 4-hydroxybutyrate-CoA synthetase in the CO2 fixation cycle in thermoacidophilic archaea.J Biol Chem. 2013 Feb 8;288(6):4012-22. doi: 10.1074/jbc.M112.413195. Epub 2012 Dec 20. J Biol Chem. 2013. PMID: 23258541 Free PMC article.
-
Conversion of 4-hydroxybutyrate to acetyl coenzyme A and its anapleurosis in the Metallosphaera sedula 3-hydroxypropionate/4-hydroxybutyrate carbon fixation pathway.Appl Environ Microbiol. 2014 Apr;80(8):2536-45. doi: 10.1128/AEM.04146-13. Epub 2014 Feb 14. Appl Environ Microbiol. 2014. PMID: 24532060 Free PMC article.
References
-
- Alber BE, Kung JW, Fuchs G. 2008. 3-Hydroxypropionyl-coenzyme A synthetase from Metallosphaera sedula, an enzyme involved in autotrophic CO2 fixation. J. Bacteriol. 190: 1383–1389 doi:10.1128/JB.01593-07 - DOI - PMC - PubMed
-
- Allen SHG, Kellermeyer R, Stjernholm R, Jacobson B, Wood HG. 1963. The isolation, purification, and properties of methylmalonyl racemase. J. Biol. Chem. 238: 1637–1642 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

