Synthesis of short-chain diols and unsaturated alcohols from secondary alcohol substrates by the Rieske nonheme mononuclear iron oxygenase MdpJ
- PMID: 22752178
- PMCID: PMC3416598
- DOI: 10.1128/AEM.01434-12
Synthesis of short-chain diols and unsaturated alcohols from secondary alcohol substrates by the Rieske nonheme mononuclear iron oxygenase MdpJ
Abstract
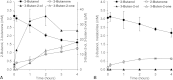
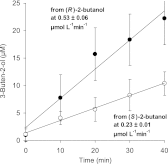
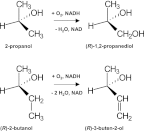
The Rieske nonheme mononuclear iron oxygenase MdpJ of the fuel oxygenate-degrading bacterial strain Aquincola tertiaricarbonis L108 has been described to attack short-chain tertiary alcohols via hydroxylation and desaturation reactions. Here, we demonstrate that also short-chain secondary alcohols can be transformed by MdpJ. Wild-type cells of strain L108 converted 2-propanol and 2-butanol to 1,2-propanediol and 3-buten-2-ol, respectively, whereas an mdpJ knockout mutant did not show such activity. In addition, wild-type cells converted 3-methyl-2-butanol and 3-pentanol to the corresponding desaturation products 3-methyl-3-buten-2-ol and 1-penten-3-ol, respectively. The enzymatic hydroxylation of 2-propanol resulted in an enantiomeric excess of about 70% for the (R)-enantiomer, indicating that this reaction was favored. Likewise, desaturation of (R)-2-butanol to 3-buten-2-ol was about 2.3-fold faster than conversion of the (S)-enantiomer. The biotechnological potential of MdpJ for the synthesis of enantiopure short-chain alcohols and diols as building block chemicals is discussed.
Figures


References
-
- Aslett D, Haas J, Hyman M. 2011. Identification of tertiary butyl alcohol (TBA)-utilizing organisms in BioGAC reactors using 13C-DNA stable isotope probing. Biodegradation 22: 961–972 - PubMed
-
- Balmer E, Germain A, Jackson WP, Lygo B. 1993. Larger scale preparation of optically-active allylic alcohols. J. Chem. Soc. Perkin Trans. 1: 399–400
-
- Bastida F, et al. 2010. Elucidating MTBE degradation in a mixed consortium using a multidisciplinary approach. FEMS Microbiol. Ecol. 73: 370–384 - PubMed
-
- Batie C, La Haie JE, Ballou DP. 1987. Purification and characterization of phthalate oxygenase and phthalate oxygenase reductase from Pseudomonas cepacia. J. Biol. Chem. 262: 1510–1518 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

