The velocity of cardiac sarcomere shortening: mechanisms and implications
- PMID: 22752243
- PMCID: PMC3568939
- DOI: 10.1007/s10974-012-9310-0
The velocity of cardiac sarcomere shortening: mechanisms and implications
Abstract
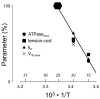
A classical paper published by Michael Barany almost 50 years ago demonstrated a tight correlation between the mechanical parameter of maximal velocity of shortening and the biochemical parameter of myosin ATPase activity in a wide spectrum of species. Here, we review the determinants of muscle dynamics by mechanical load and the relation between sarcomere shortening velocity and cross-bridge dynamics in rat myocardium containing a range of fast and slow myosin. Observations from molecular level to mechanics of the intact human heart suggest that cardiac actin-myosin kinetic properties are matched so as to optimize myocardial strain rate and allow for the maximum rate of hydraulic energy output observed during ejection in the whole ventricle.
Figures

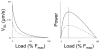
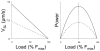


References
-
- Bacharach SL, Green MV, Borer JS, Hyde JE, Farkas SP, Johnston GS. Left-Ventricular Peak Ejection Rate, Filling Rate, and Ejection Fraction -Frame Rate Requirements at Rest and Exercise -Concise Communication. Journal of Nuclear Medicine. 1979;20:189–193. - PubMed
-
- Backx PH, ter Keurs HEDJ. Fluorescent properties of rat cardiac trabeculae microinjected with fura-2 salt. Am J Physiol. 1993;264:H1098–H1110. - PubMed
-
- Biesiadecki BJ, Kobayashi T, Walker JS, John SR, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100:1486–1493. - PubMed
-
- Burkhoff D, Sugiura S, Yue DT, Sagawa K. Contractility-dependent curvilinearity of end-systolic pressure-volume relations. Am J Physiol. 1987;252:H1218–H1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

