Dysregulation of fatty acid synthesis and glycolysis in non-Hodgkin lymphoma
- PMID: 22752304
- PMCID: PMC3406848
- DOI: 10.1073/pnas.1205995109
Dysregulation of fatty acid synthesis and glycolysis in non-Hodgkin lymphoma
Abstract
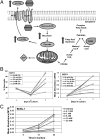
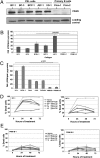
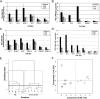
The metabolic differences between B-NHL and primary human B cells are poorly understood. Among human B-cell non-Hodgkin lymphomas (B-NHL), primary effusion lymphoma (PEL) is a unique subset that is linked to infection with Kaposi's sarcoma-associated herpesvirus (KSHV). We report that the metabolic profiles of primary B cells are significantly different from that of PEL. Compared with primary B cells, both aerobic glycolysis and fatty acid synthesis (FAS) are up-regulated in PEL and other types of nonviral B-NHL. We found that aerobic glycolysis and FAS occur in a PI3K-dependent manner and appear to be interdependent. PEL overexpress the fatty acid synthesizing enzyme, FASN, and both PEL and other B-NHL were much more sensitive to the FAS inhibitor, C75, than primary B cells. Our findings suggest that FASN may be a unique candidate for molecular targeted therapy against PEL and other B-NHL.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Boulanger E, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005;23:4372–4380. - PubMed
-
- Wang L, et al. The Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 2004;64:2774–2781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 AA017376/AA/NIAAA NIH HHS/United States
- R01 CA163217/CA/NCI NIH HHS/United States
- P30DK034987/DK/NIDDK NIH HHS/United States
- T32-CA09156/CA/NCI NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- AA017376/AA/NIAAA NIH HHS/United States
- CA163217/CA/NCI NIH HHS/United States
- T32 CA071341/CA/NCI NIH HHS/United States
- T32-AI007419/AI/NIAID NIH HHS/United States
- U01 ES019472/ES/NIEHS NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
- DK056350/DK/NIDDK NIH HHS/United States
- CA123350/CA/NCI NIH HHS/United States
- R01 CA096500/CA/NCI NIH HHS/United States
- T32 AI007151/AI/NIAID NIH HHS/United States
- DE018304/DE/NIDCR NIH HHS/United States
- T32 AI007419/AI/NIAID NIH HHS/United States
- T32AI007151/AI/NIAID NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- R00 AA017376/AA/NIAAA NIH HHS/United States
- CA096500/CA/NCI NIH HHS/United States
- R01 CA123350/CA/NCI NIH HHS/United States
- ES019472/ES/NIEHS NIH HHS/United States
- R01 DE018304/DE/NIDCR NIH HHS/United States
- T32-CA071341/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

