Evidence for the ubiquitin protease UBP43 as an antineoplastic target
- PMID: 22752428
- PMCID: PMC3438286
- DOI: 10.1158/1535-7163.MCT-12-0248
Evidence for the ubiquitin protease UBP43 as an antineoplastic target
Abstract
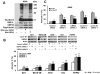
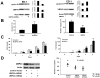
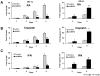
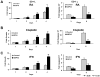
New pharmacologic targets are needed for lung cancer. One candidate pathway to target is composed of the E1-like ubiquitin-activating enzyme (UBE1L) that associates with interferon-stimulated gene 15 (ISG15), which complexes with and destabilizes cyclin D1. Ubiquitin protease 43 (UBP43/USP18) removes ISG15 from conjugated proteins. This study reports that gain of UBP43 stabilized cyclin D1, but not other D-type cyclins or cyclin E. This depended on UBP43 enzymatic activity; an enzymatically inactive UBP43 did not affect cyclin D1 stability. As expected, small interfering RNAs that reduced UBP43 expression also decreased cyclin D1 levels and increased apoptosis in a panel of lung cancer cell lines. Forced cyclin D1 expression rescued UBP43 apoptotic effects, which highlighted the importance of cyclin D1 in conferring this. Short hairpin RNA-mediated reduction of UBP43 significantly increased apoptosis and reduced murine lung cancer growth in vitro and in vivo after transplantation of these cells into syngeneic mice. These cells also exhibited increased response to all-trans-retinoic acid, interferon, or cisplatin treatments. Notably, gain of UBP43 expression antagonized these effects. Normal-malignant human lung tissue arrays were examined independently for UBP43, cyclin D1, and cyclin E immunohistochemical expression. UBP43 was significantly (P < 0.01) increased in the malignant versus normal lung. A direct relationship was found between UBP43 and cyclin D1 (but not cyclin E) expression. Differential UBP43 expression was independently detected in a normal-malignant tissue array with diverse human cancers. Taken together, these findings uncovered UBP43 as a previously unrecognized antineoplastic target.
©2012 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparity on premature deaths. CA Cancer J Clin. 2011;61:212–36. - PubMed
-
- Dragnev KH, Stover D, Dmitrovsky E. Lung cancer prevention: the guidelines. Chest. 2003;123:60S–71S. - PubMed
-
- Petty WJ, Dragnev KH, Dmitrovsky E. Cyclin D1 as a target for chemoprevention. Lung Cancer. 2003;41:S155–61. - PubMed
-
- Lonardo F, Rusch V, Langenfeld J, Dmitrovsky E, Klimstra DS. Overexpression of cyclins D1 and E is frequent in bronchial preneoplasia and precedes squamous cell carcinoma development. Cancer Res. 1999;59:2470–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

