Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction
- PMID: 22752632
- PMCID: PMC3468469
- DOI: 10.1152/ajpheart.00366.2012
Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction
Abstract
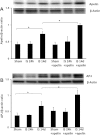
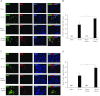
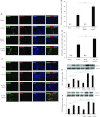
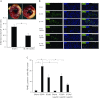
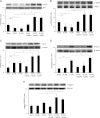
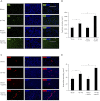
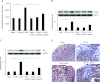
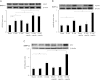
Apelin is an endogenous ligand for the angiotensin-like 1 receptor (APJ) and has beneficial effects against myocardial ischemia-reperfusion injury. Little is known about the role of apelin in the homing of vascular progenitor cells (PCs) and cardiac functional recovery postmyocardial infarction (post-MI). The present study investigated whether apelin affects PC homing to the infarcted myocardium, thereby mediating repair and functional recovery post-MI. Mice were infarcted by coronary artery ligation, and apelin-13 (1 mg·kg(-1)·day(-1)) was injected for 3 days before MI and for 14 days post-MI. Homing of vascular PCs [CD133(+)/c-Kit(+)/Sca1(+), CD133(+)/stromal cell-derived factor (SDF)-1α(+), and CD133(+)/CXC chemokine receptor (CXCR)-4(+)] into the ischemic area was examined. Myocardial Akt, endothelial nitric oxide synthase (eNOS), VEGF, jagged1, notch3, SDF-1α, and CXCR-4 expression were assessed at 24 h and 14 days post-MI. Functional analyses were performed on day 14 post-MI. Mice that received apelin-13 treatment demonstrated upregulation of SDF-1α/CXCR-4 expression and dramatically increased the number of CD133(+)/c-Kit(+)/Sca1(+), CD133(+)/SDF-1α(+), and c-Kit(+)/CXCR-4(+) cells in infarcted hearts. Apelin-13 also significantly increased Akt and eNOS phosphorylation and upregulated VEGF, jagged1, and notch3 expression in ischemic hearts. This was accompanied by a significant reduction of myocardial apoptosis. Furthermore, treatment with apelin-13 promoted myocardial angiogenesis and attenuated cardiac fibrosis and hypertrophy together with a significant improvement of cardiac function at 14 days post-MI. Apelin-13 increases angiogenesis and improves cardiac repair post-MI by a mechanism involving the upregulation of SDF-1α/CXCR-4 and homing of vascular PCs.
Figures

Similar articles
-
Overexpression of angiopoietin-1 increases CD133+/c-kit+ cells and reduces myocardial apoptosis in db/db mouse infarcted hearts.PLoS One. 2012;7(4):e35905. doi: 10.1371/journal.pone.0035905. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558265 Free PMC article.
-
Post-infarct treatment with [Pyr(1)]apelin-13 improves myocardial function by increasing neovascularization and overexpression of angiogenic growth factors in rats.Eur J Pharmacol. 2015 Aug 15;761:101-8. doi: 10.1016/j.ejphar.2015.04.034. Epub 2015 May 1. Eur J Pharmacol. 2015. PMID: 25936512
-
Myocardial injection of apelin-overexpressing bone marrow cells improves cardiac repair via upregulation of Sirt3 after myocardial infarction.PLoS One. 2013 Sep 6;8(9):e71041. doi: 10.1371/journal.pone.0071041. eCollection 2013. PLoS One. 2013. PMID: 24039710 Free PMC article.
-
SDF-1α as a therapeutic stem cell homing factor in myocardial infarction.Pharmacol Ther. 2011 Jan;129(1):97-108. doi: 10.1016/j.pharmthera.2010.09.011. Epub 2010 Oct 20. Pharmacol Ther. 2011. PMID: 20965212 Review.
-
Genetically manipulated progenitor/stem cells restore function to the infarcted heart via the SDF-1α/CXCR4 signaling pathway.Prog Mol Biol Transl Sci. 2012;111:265-84. doi: 10.1016/B978-0-12-398459-3.00012-5. Prog Mol Biol Transl Sci. 2012. PMID: 22917235 Review.
Cited by
-
Cardioprotective apelin effects and the cardiac-renal axis: review of existing science and potential therapeutic applications of synthetic and native regulated apelin.J Hum Hypertens. 2019 Jun;33(6):429-435. doi: 10.1038/s41371-019-0163-5. Epub 2019 Jan 18. J Hum Hypertens. 2019. PMID: 30659278 Review.
-
TIMP3 interplays with apelin to regulate cardiovascular metabolism in hypercholesterolemic mice.Mol Metab. 2015 Aug 6;4(10):741-52. doi: 10.1016/j.molmet.2015.07.007. eCollection 2015 Oct. Mol Metab. 2015. PMID: 26500845 Free PMC article.
-
Apelin13/APJ promotes proliferation of colon carcinoma by activating Notch3 signaling pathway.Oncotarget. 2017 Oct 13;8(60):101697-101706. doi: 10.18632/oncotarget.21904. eCollection 2017 Nov 24. Oncotarget. 2017. PMID: 29254197 Free PMC article.
-
The endoplasmic reticulum stress-autophagy pathway is involved in apelin-13-induced cardiomyocyte hypertrophy in vitro.Acta Pharmacol Sin. 2017 Dec;38(12):1589-1600. doi: 10.1038/aps.2017.97. Epub 2017 Jul 27. Acta Pharmacol Sin. 2017. PMID: 28748915 Free PMC article.
-
Protective effects of Apelin-13 on nicotine-induced H9c2 cardiomyocyte apoptosis and oxidative stress.Tob Induc Dis. 2025 Mar 18;23. doi: 10.18332/tid/201400. eCollection 2025. Tob Induc Dis. 2025. PMID: 40104400 Free PMC article.
References
-
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362: 697–703, 2003 - PubMed
-
- Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, Madeddu P. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res 104: 1095–1102, 2009 - PMC - PubMed
-
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 108: 2212–2218, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

