Including all individuals is not enough: lessons for intention-to-treat analysis
- PMID: 22752633
- PMCID: PMC3428470
- DOI: 10.1177/1740774512450098
Including all individuals is not enough: lessons for intention-to-treat analysis
Abstract
Background: Intention-to-treat (ITT) analysis requires all randomised individuals to be included in the analysis in the groups to which they were randomised. However, there is confusion about how ITT analysis should be performed in the presence of missing outcome data.
Purposes: To explain, justify, and illustrate an ITT analysis strategy for randomised trials with incomplete outcome data.
Methods: We consider several methods of analysis and compare their underlying assumptions, plausibility, and numbers of individuals included. We illustrate the ITT analysis strategy using data from the UK700 trial in the management of severe mental illness.
Results: Depending on the assumptions made about the missing data, some methods of analysis that include all randomised individuals may be less valid than methods that do not include all randomised individuals. Furthermore, some methods of analysis that include all randomised individuals are essentially equivalent to methods that do not include all randomised individuals.
Limitations: This work assumes that the aim of analysis is to obtain an accurate estimate of the difference in outcome between randomised groups and not to obtain a conservative estimate with bias against the experimental intervention.
Conclusions: Clinical trials should employ an ITT analysis strategy, comprising a design that attempts to follow up all randomised individuals, a main analysis that is valid under a stated plausible assumption about the missing data, and sensitivity analyses that include all randomised individuals in order to explore the impact of departures from the assumption underlying the main analysis. Following this strategy recognises the extra uncertainty arising from missing outcomes and increases the incentive for researchers to minimise the extent of missing data.
Figures

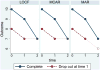
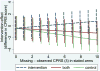
References
-
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology. 1992;21:837–841. - PubMed
-
- McKnight P, McKnight KM, Sidani S, Figueredo AJ. Missing data: A gentle introduction. The Guilford Press; New York: 2007.
-
- National Research Council. The Prevention and Treatment of Missing Data in Clinical Trials. The National Academies Press; Washington, DC: 2010. Panel on Handling Missing Data in Clinical Trials Committee on National Statistics, Division of Behavioral and Social Sciences and Education.
-
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd Wiley; Hoboken, N. J.: 2002.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

