Micro-autoradiographic assessment of cell types contributing to 2-deoxy-2-[(18)F]fluoro-D-glucose uptake during ventilator-induced and endotoxemic lung injury
- PMID: 22752654
- PMCID: PMC6391052
- DOI: 10.1007/s11307-012-0575-x
Micro-autoradiographic assessment of cell types contributing to 2-deoxy-2-[(18)F]fluoro-D-glucose uptake during ventilator-induced and endotoxemic lung injury
Abstract
Purpose: The aim of the study was to use micro-autoradiography to investigate the lung cell types responsible for 2-deoxy-2-[(18)F]fluoro-D-glucose (FDG) uptake in murine models of acute lung injury (ALI).
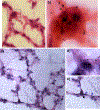
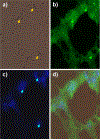
Procedures: C57/BL6 mice were studied in three groups: controls, ventilator-induced lung injury (VILI), and endotoxin. VILI was produced by high tidal volumes and zero end-expiratory pressure and endotoxin ALI, by intranasal administration. Following FDG injection, the lungs were processed and exposed to autoradiographic emulsion. Grain density over cells was used to quantify FDG uptake.
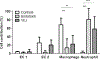
Results: Neutrophils, macrophages, and type 2 epithelial cells presented higher grain densities during VILI and endotoxin ALI than controls. Remarkably, cell grain density in specific cell types was dependent on the injury mechanism. Whereas macrophages showed high grain densities during endotoxin ALI, similar to those exhibited by neutrophils, type 2 epithelial cells demonstrated the second highest grain density (with neutrophils as the highest) during VILI.
Conclusions: In murine models of VILI and endotoxin ALI, FDG uptake occurs not only in neutrophils but also in macrophages and type 2 epithelial cells. FDG uptake by individual cell types depends on the mechanism underlying ALI.
Conflict of interest statement
Figures
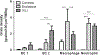


Similar articles
-
Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding.Am J Respir Cell Mol Biol. 2015 Feb;52(2):193-204. doi: 10.1165/rcmb.2013-0347OC. Am J Respir Cell Mol Biol. 2015. PMID: 25029266 Free PMC article.
-
2-deoxy-2-[18F]fluoro-D-glucose Positron Emission Tomography to Monitor Lung Inflammation and Therapeutic Response to Dexamethasone in a Murine Model of Acute Lung Injury.Mol Imaging Biol. 2023 Aug;25(4):681-691. doi: 10.1007/s11307-023-01813-w. Epub 2023 Mar 20. Mol Imaging Biol. 2023. PMID: 36941514 Free PMC article.
-
18F-fluoro-2-deoxyglucose PET informs neutrophil accumulation and activation in lipopolysaccharide-induced acute lung injury.Nucl Med Biol. 2017 May;48:52-62. doi: 10.1016/j.nucmedbio.2017.01.005. Epub 2017 Jan 17. Nucl Med Biol. 2017. PMID: 28237630 Free PMC article.
-
Positron emission tomography: a tool for better understanding of ventilator-induced and acute lung injury.Curr Opin Crit Care. 2011 Feb;17(1):7-12. doi: 10.1097/MCC.0b013e32834272ab. Curr Opin Crit Care. 2011. PMID: 21169828 Free PMC article. Review.
-
Mechanisms of ventilator-induced lung injury in healthy lungs.Best Pract Res Clin Anaesthesiol. 2015 Sep;29(3):301-13. doi: 10.1016/j.bpa.2015.08.004. Epub 2015 Sep 1. Best Pract Res Clin Anaesthesiol. 2015. PMID: 26643096 Review.
Cited by
-
Molecular imaging of inflammation with PET in acute and ventilator-induced lung injury.Front Physiol. 2023 Jun 29;14:1177717. doi: 10.3389/fphys.2023.1177717. eCollection 2023. Front Physiol. 2023. PMID: 37457026 Free PMC article. Review.
-
Effects of ventilation strategy on distribution of lung inflammatory cell activity.Crit Care. 2013 Aug 15;17(4):R175. doi: 10.1186/cc12854. Crit Care. 2013. PMID: 23947920 Free PMC article.
-
Pathogenesis of ventilator-induced lung injury: metabolomics analysis of the lung and plasma.Metabolomics. 2022 Aug 4;18(8):66. doi: 10.1007/s11306-022-01914-7. Metabolomics. 2022. PMID: 35925420
-
Lung [(18)F]fluorodeoxyglucose uptake and ventilation-perfusion mismatch in the early stage of experimental acute smoke inhalation.Anesthesiology. 2014 Mar;120(3):683-93. doi: 10.1097/01.anes.0000435742.04859.e8. Anesthesiology. 2014. PMID: 24051392 Free PMC article.
-
Lung metabolism during ventilator-induced lung injury: stretching the relevance of the normally aerated lung*.Crit Care Med. 2014 Apr;42(4):1010-2. doi: 10.1097/CCM.0000000000000251. Crit Care Med. 2014. PMID: 24633113 Free PMC article. No abstract available.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693 - PubMed
-
- Musch G, Venegas JG, Bellani G, Winkler T, Schroeder T, Petersen B, Harris RS, Melo MF (2007) Regional gas exchange and cellular metabolic activity in ventilator-induced lung injury. Anesthesiology 106:723–735 - PubMed
-
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282:54–61 - PubMed
-
- Suter PM (2006) Lung inflammation in ARDS—friend or foe? N Engl J Med 354:1739–1742 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

