Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer
- PMID: 22752690
- PMCID: PMC3553405
- DOI: 10.1007/s10637-012-9842-6
Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer
Abstract
Introduction: This multicenter, open-label, phase II study was carried out to compare the efficacy and safety of cilengitide (EMD 121974), a selective inhibitor of the cell-surface integrins αVβ3 and αVβ5, with that of docetaxel in patients with advanced non-small-cell lung cancer (NSCLC).
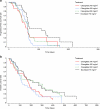
Methods: Patients (n = 140) with advanced NSCLC who had failed first-line chemotherapy were randomized to cilengitide 240, 400, or 600 mg/m(2) twice weekly, or docetaxel 75 mg/m(2) once every 3 weeks for eight cycles. Non-progressing patients could continue cilengitide for up to 1 year. The primary endpoint was progression-free survival (PFS). No statistical tests were performed since the study was exploratory in nature and the number of patients enrolled was relatively small.
Results: Median PFS was 54, 63, 63, and 67 days for cilengitide 240, 400, and 600 mg/m(2), and docetaxel 75 mg/m(2), respectively. One-year survival rates were 13 %, 13 %, 29 %, and 27 %, respectively. The response rate (partial response only) with docetaxel was 15 %. No responses were reported in any cilengitide arm. The most frequent grade 3/4 treatment-related adverse events in the docetaxel group were leukopenia and neutropenia (experienced by 13 % of patients). Hematologic toxicity of this severity did not occur in cilengitide-treated patients.
Conclusion: With the highest dose of cilengitide (600 mg/m(2)), median PFS and 1-year survival were similar to those in patients treated with docetaxel 75 mg/m(2) and there were fewer grade 3/4 treatment-related adverse events.
Figures
References
-
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. - PubMed
-
- Dancey J, Shepherd FA, Gralla RJ, Kim YS. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung Cancer. 2004;43:183–194. doi: 10.1016/j.lungcan.2003.09.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical