Neurons and β-cells of the pancreas express connexin36, forming gap junction channels that exhibit strong cationic selectivity
- PMID: 22752717
- PMCID: PMC3626077
- DOI: 10.1007/s00232-012-9445-3
Neurons and β-cells of the pancreas express connexin36, forming gap junction channels that exhibit strong cationic selectivity
Abstract
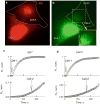
We examined the permeability of connexin36 (Cx36) homotypic gap junction (GJ) channels, expressed in neurons and β-cells of the pancreas, to dyes differing in molecular mass and net charge. Experiments were performed in HeLa cells stably expressing Cx36 tagged with EGFP by combining a dual whole-cell voltage clamp and fluorescence imaging. To assess the permeability of the single GJ channel (P(γ)), we used a dual-mode excitation of fluorescent dyes that allowed us to measure cell-to-cell dye transfer at levels not resolvable using whole-field excitation solely. We demonstrate that P(γ) of Cx36 for cationic dyes (EAM-1⁺ and EAM-2⁺) is ~10-fold higher than that for an anionic dye of the same net charge and similar molecular mass, Alexa fluor-350 (AFl-350⁻). In addition, P(γ) for Lucifer yellow (LY²⁻) is approximately fourfold smaller than that for AFl-350⁻, which suggests that the higher negativity of LY²⁻ significantly reduces permeability. The P(γ) of Cx36 for AFl-350 is approximately 358, 138, 23 and four times smaller than the P(γ)s of Cx43, Cx40, Cx45, and Cx57, respectively. In contrast, it is 6.5-fold higher than the P(γ) of mCx30.2, which exhibits a smaller single-channel conductance. Thus, Cx36 GJs are highly cation-selective and should exhibit relatively low permeability to numerous vital negatively charged metabolites and high permeability to K⁺, a major charge carrier in cell-cell communication.
Figures



Similar articles
-
Permeability of homotypic and heterotypic gap junction channels formed of cardiac connexins mCx30.2, Cx40, Cx43, and Cx45.Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1729-36. doi: 10.1152/ajpheart.00234.2007. Epub 2007 Jun 8. Am J Physiol Heart Circ Physiol. 2007. PMID: 17557922 Free PMC article.
-
Gap junction permeability: selectivity for anionic and cationic probes.Am J Physiol Cell Physiol. 2011 Mar;300(3):C600-9. doi: 10.1152/ajpcell.00316.2010. Epub 2010 Dec 9. Am J Physiol Cell Physiol. 2011. PMID: 21148413 Free PMC article.
-
Gating, permselectivity and pH-dependent modulation of channels formed by connexin57, a major connexin of horizontal cells in the mouse retina.J Physiol. 2009 Jul 1;587(Pt 13):3251-69. doi: 10.1113/jphysiol.2009.171496. Epub 2009 May 11. J Physiol. 2009. PMID: 19433576 Free PMC article.
-
Modulation of metabolic communication through gap junction channels by transjunctional voltage; synergistic and antagonistic effects of gating and ionophoresis.Biochim Biophys Acta. 2012 Aug;1818(8):1884-94. doi: 10.1016/j.bbamem.2011.09.001. Epub 2011 Sep 10. Biochim Biophys Acta. 2012. PMID: 21930112 Free PMC article. Review.
-
Defining the factors that affect solute permeation of gap junction channels.Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):96-101. doi: 10.1016/j.bbamem.2017.07.002. Epub 2017 Jul 6. Biochim Biophys Acta Biomembr. 2018. PMID: 28690048 Free PMC article. Review.
Cited by
-
Connexins-Based Hemichannels/Channels and Their Relationship with Inflammation, Seizures and Epilepsy.Int J Mol Sci. 2019 Nov 27;20(23):5976. doi: 10.3390/ijms20235976. Int J Mol Sci. 2019. PMID: 31783599 Free PMC article. Review.
-
Connexin36 localization to pinealocytes in the pineal gland of mouse and rat.Eur J Neurosci. 2017 Jun;45(12):1594-1605. doi: 10.1111/ejn.13602. Epub 2017 May 25. Eur J Neurosci. 2017. PMID: 28474748 Free PMC article.
-
Gap junctions coordinate the propagation of glycogenolysis induced by norepinephrine in the pineal gland.J Neurochem. 2019 Dec;151(5):558-569. doi: 10.1111/jnc.14846. Epub 2019 Oct 20. J Neurochem. 2019. PMID: 31381153 Free PMC article.
-
Midbody: From the Regulator of Cytokinesis to Postmitotic Signaling Organelle.Medicina (Kaunas). 2018 Jul 30;54(4):53. doi: 10.3390/medicina54040053. Medicina (Kaunas). 2018. PMID: 30344284 Free PMC article. Review.
-
The Role of cAMP in Beta Cell Stimulus-Secretion and Intercellular Coupling.Cells. 2021 Jul 1;10(7):1658. doi: 10.3390/cells10071658. Cells. 2021. PMID: 34359828 Free PMC article. Review.
References
-
- Bednarczyk D, Mash EA, Aavula BR, Wright SH. NBD-TMA: a novel fluorescent substrate of the peritubular organic cation transporter of renal proximal tubules. Pflugers Arch. 2000;440:184–192. - PubMed
-
- Bedner P, Niessen H, Odermatt B, Kretz M, Willecke K, Harz H. Selective permeability of different connexin channels to the second messenger cyclic AMP. J Biol Chem. 2006;28:6673–6681. - PubMed
-
- Bennett MV, Verselis VK. Biophysics of gap junctions. Semin Cell Biol. 1992;3:29–47. - PubMed
-
- Bukauskas FF. Inducing de novo formation of gap junction channels. Method Mol Biol. 2001;154:379–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

