Personalized medicine using DNA biomarkers: a review
- PMID: 22752797
- PMCID: PMC3432208
- DOI: 10.1007/s00439-012-1188-9
Personalized medicine using DNA biomarkers: a review
Abstract
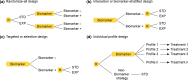
Biomarkers are of increasing importance for personalized medicine, with applications including diagnosis, prognosis, and selection of targeted therapies. Their use is extremely diverse, ranging from pharmacodynamics to treatment monitoring. Following a concise review of terminology, we provide examples and current applications of three broad categories of biomarkers-DNA biomarkers, DNA tumor biomarkers, and other general biomarkers. We outline clinical trial phases for identifying and validating diagnostic and prognostic biomarkers. Predictive biomarkers, more generally termed companion diagnostic tests predict treatment response in terms of efficacy and/or safety. We consider suitability of clinical trial designs for predictive biomarkers, including a detailed discussion of validation study designs, with emphasis on interpretation of study results. We specifically discuss the interpretability of treatment effects if a large set of DNA biomarker profiles is available and the number of therapies is identical to the number of different profiles.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

