Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI
- PMID: 22752862
- PMCID: PMC3388020
- DOI: 10.1093/cid/cis338
Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI
Abstract
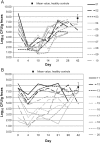
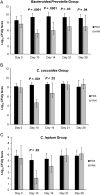
The microflora-sparing properties of fidaxomicin were examined during the conduct of a randomized clinical trial comparing vancomycin 125 mg 4 times per day versus fidaxomicin 200 mg twice per day for 10 days as treatment of Clostridium difficile infection (CDI). Fecal samples were obtained from 89 patients (45 received fidaxomicin, and 44 received vancomycin) at study entry and on days 4, 10, 14, 21, 28, and 38 for quantitative cultures for C. difficile and cytotoxin B fecal filtrate concentrations. Additionally, samples from 10 patients, each receiving vancomycin or fidaxomicin, and 10 samples from healthy controls were analyzed by quantitative real-time polymerase chain reaction with multiple group-specific primers to evaluate the impact of antibiotic treatment on the microbiome. Compared with controls, patients with CDI at study entry had counts of major microbiome components that were 2-3-log(10) colony-forming units (CFU)/g lower. In patients with CDI, fidaxomicin allowed the major components to persist, whereas vancomycin was associated with a further 2-4-log(10) CFU reduction of Bacteroides/Prevotella group organisms, which persisted to day 28 of the study, and shorter term and temporary suppression of both Clostridium coccoides and Clostridium leptum group organisms. In the posttreatment period, C. difficile counts similarly persisted in both study populations, but reappearance of toxin in fecal filtrates was observed in 28% of vancomycin-treated patient samples (29 of 94), compared with 14% of fidaxomicin-treated patient samples (13 of 91; P = .03). Similarly, 23% of vancomycin-treated patients (10 of 44) and 11% of fidaxomicin-treated patients (5 of 44) had recurrence of CDI. Whereas vancomycin and fidaxomicin are equally effective in resolving CDI symptoms, preservation of the microflora by fidaxomicin is associated with a lower likelihood of CDI recurrence.
Trial registration: ClinicalTrials.gov NCT00314951.
Figures









Similar articles
-
Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin.Clin Infect Dis. 2012 Aug;55 Suppl 2(Suppl 2):S154-61. doi: 10.1093/cid/cis462. Clin Infect Dis. 2012. PMID: 22752865 Free PMC article. Clinical Trial.
-
Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin.Clin Infect Dis. 2012 Aug;55 Suppl 2(Suppl 2):S104-9. doi: 10.1093/cid/cis357. Clin Infect Dis. 2012. PMID: 22752857 Free PMC article. Clinical Trial.
-
Vancomycin treatment's association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice.Antimicrob Agents Chemother. 2013 Feb;57(2):689-96. doi: 10.1128/AAC.00877-12. Epub 2012 Nov 12. Antimicrob Agents Chemother. 2013. PMID: 23147742 Free PMC article.
-
Fidaxomicin: a novel macrocyclic antibiotic for the treatment of Clostridium difficile infection.Am J Health Syst Pharm. 2012 Jun 1;69(11):933-43. doi: 10.2146/ajhp110371. Am J Health Syst Pharm. 2012. PMID: 22610025 Review.
-
Current and emerging management options for Clostridium difficile infection: what is the role of fidaxomicin?Clin Microbiol Infect. 2012 Dec;18 Suppl 6:28-35. doi: 10.1111/1469-0691.12012. Clin Microbiol Infect. 2012. PMID: 23121552 Review.
Cited by
-
Comparative effectiveness of treatments for recurrent Clostridioides difficile infection: a network meta-analysis of randomized controlled trials.Front Pharmacol. 2024 Oct 17;15:1430724. doi: 10.3389/fphar.2024.1430724. eCollection 2024. Front Pharmacol. 2024. PMID: 39484168 Free PMC article.
-
Clostridium difficile Infection.Microbiol Spectr. 2016 Jun;4(3):10.1128/microbiolspec.EI10-0007-2015. doi: 10.1128/microbiolspec.EI10-0007-2015. Microbiol Spectr. 2016. PMID: 27337475 Free PMC article. Review.
-
Geriatric nutritional risk index as a risk-factor for Clostridioides difficile infection relapse in elderly Japanese patients.J Rural Med. 2022 Oct;17(4):248-254. doi: 10.2185/jrm.2022-027. Epub 2022 Oct 22. J Rural Med. 2022. PMID: 36397789 Free PMC article.
-
A pilot study to assess bacterial and toxin reduction in patients with Clostridium difficile infection given fidaxomicin or vancomycin.Ann Clin Microbiol Antimicrob. 2016 Apr 12;15:22. doi: 10.1186/s12941-016-0140-6. Ann Clin Microbiol Antimicrob. 2016. PMID: 27071986 Free PMC article.
-
Fecal transplant in refractory Clostridium difficile colitis.Dtsch Arztebl Int. 2013 Feb;110(7):108-15. doi: 10.3238/arztebl.2013.0108. Epub 2013 Feb 15. Dtsch Arztebl Int. 2013. PMID: 23468820 Free PMC article.
References
-
- Olson MM, Shanholtzer CJ, Lee JT, Jr, Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect Control Hosp Epidemiol. 1994;15:371–81. - PubMed
-
- Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5:549–57. - PubMed
-
- Kelly CP, La Mont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–40. - PubMed
-
- Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–90. - PubMed
-
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

