Structural insights into the regulatory mechanism of the response regulator RocR from Pseudomonas aeruginosa in cyclic Di-GMP signaling
- PMID: 22753070
- PMCID: PMC3430337
- DOI: 10.1128/JB.00560-12
Structural insights into the regulatory mechanism of the response regulator RocR from Pseudomonas aeruginosa in cyclic Di-GMP signaling
Abstract
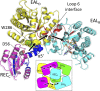
The nucleotide messenger cyclic di-GMP (c-di-GMP) plays a central role in the regulation of motility, virulence, and biofilm formation in many pathogenic bacteria. EAL domain-containing phosphodiesterases are the major signaling proteins responsible for the degradation of c-di-GMP and maintenance of its cellular level. We determined the crystal structure of a single mutant (R286W) of the response regulator RocR from Pseudomonas aeruginosa to show that RocR exhibits a highly unusual tetrameric structure arranged around a single dyad, with the four subunits adopting two distinctly different conformations. Subunits A and B adopt a conformation with the REC domain located above the c-di-GMP binding pocket, whereas subunits C and D adopt an open conformation with the REC domain swung to the side of the EAL domain. Remarkably, the access to the substrate-binding pockets of the EAL domains of the open subunits C and D are blocked in trans by the REC domains of subunits A and B, indicating that only two of the four active sites are engaged in the degradation of c-di-GMP. In conjunction with biochemical and biophysical data, we propose that the structural changes within the REC domains triggered by the phosphorylation are transmitted to the EAL domain active sites through a pathway that traverses the dimerization interfaces composed of a conserved regulatory loop and the neighboring motifs. This exquisite mechanism reinforces the crucial role of the regulatory loop and suggests that similar regulatory mechanisms may be operational in many EAL domain proteins, considering the preservation of the dimerization interface and the spatial arrangement of the regulatory domains.
Figures





Similar articles
-
The functional role of a conserved loop in EAL domain-based cyclic di-GMP-specific phosphodiesterase.J Bacteriol. 2009 Aug;191(15):4722-31. doi: 10.1128/JB.00327-09. Epub 2009 Apr 17. J Bacteriol. 2009. PMID: 19376848 Free PMC article.
-
Catalytic mechanism of cyclic di-GMP-specific phosphodiesterase: a study of the EAL domain-containing RocR from Pseudomonas aeruginosa.J Bacteriol. 2008 May;190(10):3622-31. doi: 10.1128/JB.00165-08. Epub 2008 Mar 14. J Bacteriol. 2008. PMID: 18344366 Free PMC article.
-
Mechanistic insights into c-di-GMP-dependent control of the biofilm regulator FleQ from Pseudomonas aeruginosa.Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):E209-18. doi: 10.1073/pnas.1523148113. Epub 2015 Dec 28. Proc Natl Acad Sci U S A. 2016. PMID: 26712005 Free PMC article.
-
Oligoribonuclease is a central feature of cyclic diguanylate signaling in Pseudomonas aeruginosa.Proc Natl Acad Sci U S A. 2015 Sep 8;112(36):11359-64. doi: 10.1073/pnas.1421450112. Epub 2015 Aug 24. Proc Natl Acad Sci U S A. 2015. PMID: 26305928 Free PMC article.
-
c-di-GMP signalling and the regulation of developmental transitions in streptomycetes.Nat Rev Microbiol. 2015 Dec;13(12):749-60. doi: 10.1038/nrmicro3546. Epub 2015 Oct 26. Nat Rev Microbiol. 2015. PMID: 26499894 Review.
Cited by
-
PafS Containing GGDEF-Domain Regulates Life Activities of Pseudomonas glycinae MS82.Microorganisms. 2022 Nov 26;10(12):2342. doi: 10.3390/microorganisms10122342. Microorganisms. 2022. PMID: 36557595 Free PMC article.
-
Phosphorylation chemistry of the Bordetella PlrSR TCS and its contribution to bacterial persistence in the lower respiratory tract.Mol Microbiol. 2023 Feb;119(2):174-190. doi: 10.1111/mmi.15019. Epub 2023 Jan 16. Mol Microbiol. 2023. PMID: 36577696 Free PMC article.
-
Functional and Pangenomic Exploration of Roc Two-Component Regulatory Systems Identifies Novel Players Across Pseudomonas Species.Mol Microbiol. 2025 May;123(5):439-453. doi: 10.1111/mmi.15357. Epub 2025 Mar 14. Mol Microbiol. 2025. PMID: 40087830 Free PMC article.
-
Inherent regulation of EAL domain-catalyzed hydrolysis of second messenger cyclic di-GMP.J Biol Chem. 2014 Mar 7;289(10):6978-6990. doi: 10.1074/jbc.M113.516195. Epub 2014 Jan 22. J Biol Chem. 2014. PMID: 24451384 Free PMC article.
-
Inhibition of P. aeruginosa c-di-GMP phosphodiesterase RocR and swarming motility by a benzoisothiazolinone derivative.Chem Sci. 2016 Sep 1;7(9):6238-6244. doi: 10.1039/c6sc02103d. Epub 2016 Jun 16. Chem Sci. 2016. PMID: 30034764 Free PMC article.
References
-
- Barends TRM, et al. 2009. Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 459:1015–1018 - PubMed
-
- Chang AL, et al. 2001. Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry 40:3420–3426 - PubMed
-
- Cho HS, et al. 2000. NMR structure of activated CheY. J. Mol. Biol. 297:543–551 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

