Associations among genotype, clinical phenotype, and intracellular localization of trafficking proteins in ARC syndrome
- PMID: 22753090
- PMCID: PMC3746110
- DOI: 10.1002/humu.22155
Associations among genotype, clinical phenotype, and intracellular localization of trafficking proteins in ARC syndrome
Abstract
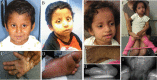
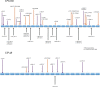
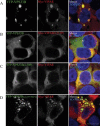
Arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome is a rare autosomal recessive multisystem disorder caused by mutations in vacuolar protein sorting 33 homologue B (VPS33B) and VPS33B interacting protein, apical-basolateral polarity regulator (VIPAR). Cardinal features of ARC include congenital joint contractures, renal tubular dysfunction, cholestasis, severe failure to thrive, ichthyosis, and a defect in platelet alpha-granule biogenesis. Most patients with ARC do not survive past the first year of life. We report two patients presenting with a mild ARC phenotype, now 5.5 and 3.5 years old. Both patients were compound heterozygotes with the novel VPS33B donor splice-site mutation c.1225+5G>C in common. Immunoblotting and complementary DNA analysis suggest expression of a shorter VPS33B transcript, and cell-based assays show that c.1225+5G>C VPS33B mutant retains some ability to interact with VIPAR (and thus partial wild-type function). This study provides the first evidence of genotype-phenotype correlation in ARC and suggests that VPS33B c.1225+5G>C mutation predicts a mild ARC phenotype. We have established an interactive online database for ARC (https://grenada.lumc.nl/LOVD2/ARC) comprising all known variants in VPS33B and VIPAR. Also included in the database are 15 novel pathogenic variants in VPS33B and five in VIPAR.
© 2012 Wiley Periodicals, Inc.
Figures
Similar articles
-
Identification of novel mutations in the VPS33B gene involved in arthrogryposis, renal dysfunction, and cholestasis syndrome.Clin Genet. 2015 Jul;88(1):80-4. doi: 10.1111/cge.12442. Epub 2014 Aug 7. Clin Genet. 2015. PMID: 24917129
-
One case of arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome featuring an incomplete and mild phenotype.BMC Nephrol. 2022 Jun 27;23(1):228. doi: 10.1186/s12882-022-02851-2. BMC Nephrol. 2022. PMID: 35761207 Free PMC article.
-
ARC syndrome with high GGT cholestasis caused by VPS33B mutations.World J Gastroenterol. 2014 Apr 28;20(16):4830-4. doi: 10.3748/wjg.v20.i16.4830. World J Gastroenterol. 2014. PMID: 24782640 Free PMC article.
-
Arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome: from molecular genetics to clinical features.Ital J Pediatr. 2014 Sep 20;40:77. doi: 10.1186/s13052-014-0077-3. Ital J Pediatr. 2014. PMID: 25239142 Free PMC article. Review.
-
VIPAS39 related arthrogryposis-renal dysfunction-cholestasis syndrome-case report and systematic review.Orphanet J Rare Dis. 2024 Dec 30;19(1):496. doi: 10.1186/s13023-024-03486-2. Orphanet J Rare Dis. 2024. PMID: 39736737 Free PMC article.
Cited by
-
Mild Phenotype of Arthrogryposis, Renal Dysfunction, and Cholestasis Syndrome 1 Caused by a Novel VPS33B Variant.Front Genet. 2022 Feb 25;13:796759. doi: 10.3389/fgene.2022.796759. eCollection 2022. Front Genet. 2022. PMID: 35281816 Free PMC article.
-
VPS33B and VIPAR are essential for epidermal lamellar body biogenesis and function.Biochim Biophys Acta Mol Basis Dis. 2018 May;1864(5 Pt A):1609-1621. doi: 10.1016/j.bbadis.2018.01.028. Epub 2018 Jan 31. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29409756 Free PMC article.
-
A Novel Mutation of VPS33B Gene Associated with Incomplete Arthrogryposis-Renal Dysfunction-Cholestasis Phenotype.Case Rep Genet. 2020 Sep 24;2020:8872294. doi: 10.1155/2020/8872294. eCollection 2020. Case Rep Genet. 2020. PMID: 33029437 Free PMC article.
-
Novel gene mutations in three Japanese patients with ARC syndrome associated mild phenotypes: a case series.J Med Case Rep. 2022 Feb 13;16(1):60. doi: 10.1186/s13256-022-03279-w. J Med Case Rep. 2022. PMID: 35151346 Free PMC article.
-
Hanging on by a thread: a rare case of secondary pseudoainhum.BMJ Case Rep. 2016 Feb 2;2016:bcr2015213854. doi: 10.1136/bcr-2015-213854. BMJ Case Rep. 2016. PMID: 26838543 Free PMC article.
References
-
- Cullinane AR, Straatman-Iwanowska A, Zaucker A, Wakabayashi Y, Bruce CK, Luo G, Rahman F, Gurakan F, Utine E, Ozkan TB, Denecke J, Vukovik J, et al. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet. 2010;42:303–312. - PMC - PubMed
-
- Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, den Dunnen JT. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:1–7. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

