Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: a key regulator of extracellular superoxide dismutase
- PMID: 22753205
- PMCID: PMC3415283
- DOI: 10.1161/HYPERTENSIONAHA.111.189571
Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: a key regulator of extracellular superoxide dismutase
Abstract
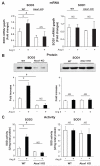
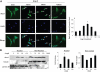
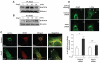
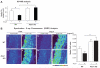
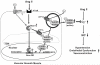
Extracellular superoxide dismutase (SOD3) is a secretory copper enzyme involved in protecting angiotensin II (Ang II)-induced hypertension. We found previously that Ang II upregulates SOD3 expression and activity as a counterregulatory mechanism; however, underlying mechanisms are unclear. Antioxidant 1 (Atox1) is shown to act as a copper-dependent transcription factor, as well as a copper chaperone, for SOD3 in vitro, but its role in Ang II-induced hypertension in vivo is unknown. Here we show that Ang II infusion increases Atox1 expression, as well as SOD3 expression and activity, in aortas of wild-type mice, which are inhibited in mice lacking Atox1. Accordingly, Ang II increases vascular superoxide production, reduces endothelium-dependent vasodilation, and increases vasoconstriction in mesenteric arteries to a greater extent in Atox1(-/-) than in wild-type mice. This contributes to augmented hypertensive response to Ang II in Atox1(-/-) mice. In cultured vascular smooth muscle cells, Ang II promotes translocation of Atox1 to the nucleus, thereby increasing SOD3 transcription by binding to Atox1-responsive element in the SOD3 promoter. Furthermore, Ang II increases Atox1 binding to the copper exporter ATP7A, which obtains copper from Atox1, as well as translocation of ATP7A to plasma membranes, where it colocalizes with SOD3. As its consequence, Ang II decreases vascular copper levels, which is inhibited in Atox1(-/-) mice. In summary, Atox1 functions to prevent Ang II-induced endothelial dysfunction and hypercontraction in resistant vessels, as well as hypertension, in vivo by reducing extracellular superoxide levels via increasing vascular SOD3 expression and activity.
Figures
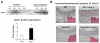
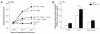
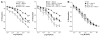

Comment in
-
Antioxidant 1 in hypertension: more than just a copper chaperone.Hypertension. 2012 Aug;60(2):285-7. doi: 10.1161/HYPERTENSIONAHA.112.191304. Epub 2012 Jul 2. Hypertension. 2012. PMID: 22753212 Free PMC article. No abstract available.
References
-
- Chu Y, Alwahdani A, Iida S, Lund DD, Faraci FM, Heistad DD. Vascular effects of the human extracellular superoxide dismutase R213G variant. Circulation. 2005;112:1047–1053. - PubMed
-
- Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. - PubMed
-
- Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

