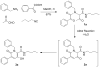
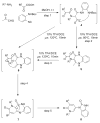
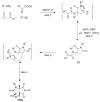
Ugi/aldol sequence: expeditious entry to several families of densely substituted nitrogen heterocycles
- PMID: 22753341
- PMCID: PMC3517102
- DOI: 10.1002/anie.201202575
Ugi/aldol sequence: expeditious entry to several families of densely substituted nitrogen heterocycles
Abstract
All it takes is the Aldol: Nitrogen containing heterocycles have been assembled by means of unprecedented domino processes, designed to take advantage of diversity assembly via strategically decorated Ugi products. The aldol reaction is the second common denominator which enables sequences of up to 5 steps in one pot producing unique molecular architecture in rapid fashion.
Figures
References
-
- Ruijter E, Scheffelaar R, Orru RVA. Angew Chem. 2011;123:6358–6371. - PubMed
- Angew Chem Int Ed Engl. 2011;50:6234–6246. - PubMed
- Bonne D, Dekhane M, Zhu J. Angew Chem. 2007;119:2537–2540. - PubMed
- Angew Chem Int Ed Engl. 2007;46:2485–2488. - PubMed
- Bienaymé H, Bouzid K. Angew Chem. 1998;110:2349–1352. - PubMed
- Angew Chem Int Ed Engl. 1998;37:2234–2237. - PubMed
-
- Dömling A. Chem Rev. 2006;106:17–89. - PubMed
- Zhu J, Bienaymé H, editors. Multicomponent Reactions. WILEY-VCH; Weinheim, Germany: 2005.
-
- Schreiber SL. Science. 2000;287:1964–1969. - PubMed
-
- Hulme C, Dietrich J. Mol Divers. 2009;13:195–207. - PubMed
- El Kaim L, Grimaud L. Tetrahedron. 2009;65:2153–2171.
-
-
For a recent overview see: Ayaz M, De Moliner F, Dietrich J, Hulme C. In: Evolution of Isocyanide Chemistry. Nenajdenko V, editor. WILEY-VCH; Weinheim, Germany: in press.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources