Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity
- PMID: 22753374
- PMCID: PMC3418755
- DOI: 10.1128/IAI.00123-12
Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity
Abstract
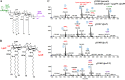
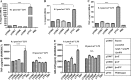
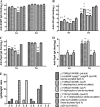
Lipid A is a key component of the outer membrane of Gram-negative bacteria and stimulates proinflammatory responses via the Toll-like receptor 4 (TLR4)-MD2-CD14 pathway. Its endotoxic activity depends on the number and length of acyl chains and its phosphorylation state. In Salmonella enterica serovar Typhimurium, removal of the secondary laurate or myristate chain in lipid A results in bacterial attenuation and growth defects in vitro. However, the roles of the two lipid A phosphate groups in bacterial virulence and immunogenicity remain unknown. Here, we used an S. Typhimurium msbB pagL pagP lpxR mutant, carrying penta-acylated lipid A, as the parent strain to construct a series of mutants synthesizing 1-dephosphorylated, 4'-dephosphorylated, or nonphosphorylated penta-acylated lipid A. Dephosphorylated mutants exhibited increased sensitivity to deoxycholate and showed increased resistance to polymyxin B. Removal of both phosphate groups severely attenuated the mutants when administered orally to BALB/c mice, but the mutants colonized the lymphatic tissues and were sufficiently immunogenic to protect the host from challenge with wild-type S. Typhimurium. Mice receiving S. Typhimurium with 1-dephosphorylated or nonphosphorylated penta-acylated lipid A exhibited reduced levels of cytokines. Attenuated and dephosphorylated Salmonella vaccines were able to induce adaptive immunity against heterologous (PspA of Streptococcus pneumoniae) and homologous antigens (lipopolysaccharide [LPS] and outer membrane proteins [OMPs]).
Figures

References
-
- Bainbridge BW, Darveau RP. 2001. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol. Scand. 59:131–138 - PubMed
-
- Bhat UR, Forsberg LS, Carlson RW. 1994. Structure of lipid A component of Rhizobium Leguminosarum bv Phaseoli lipopolysaccharide—unique nonphosphorylated lipid A containing 2-amino-8-deoxygluconate, galacturonate, and glucosamine. J. Biol. Chem. 269:14402–14410 - PubMed
-
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37:911–917 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

