Biofilm-infected intracerebroventricular shunts elicit inflammation within the central nervous system
- PMID: 22753376
- PMCID: PMC3418739
- DOI: 10.1128/IAI.00645-12
Biofilm-infected intracerebroventricular shunts elicit inflammation within the central nervous system
Abstract
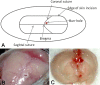
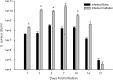
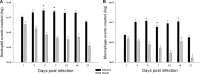
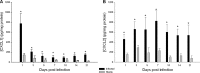
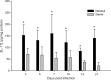
Central nervous system catheter infections are a serious complication in the treatment of hydrocephalus. These infections are commonly caused by Staphylococcus epidermidis and Staphylococcus aureus, both known to form biofilms on the catheter surface. Our objective was to generate a novel murine model of central nervous system catheter-associated biofilm infection using a clinical S. aureus isolate and characterize the nature of the inflammatory response during biofilm growth. Silicone catheters were precoated with S. aureus to facilitate bacterial attachment, whereupon infected or sterile catheters were stereotactically inserted into the lateral ventricle of the brain in C57BL/6 mice and evaluated at regular intervals through day 21 postinsertion. Animals tolerated the procedure well, with no clinical signs of illness or bacterial growth seen in the control group. Bacterial titers associated with central nervous system catheters were significantly elevated compared to those from the surrounding parenchyma, consistent with biofilm formation and minimal planktonic spread of infection. Catheter-associated bacterial burdens progressively increased, with maximal colonization achieved at day 7 postinfection. Analysis of inflammatory infiltrates by fluorescence-activated cell sorting (FACS) revealed significant macrophage and neutrophil influx, which peaked at days 3 and 5 to 7, respectively. In contrast, there were no detectable immune infiltrates associated with tissues surrounding sterile catheters. Biofilm infection led to significant increases in chemokine (CXCL1 and CCL2) and proinflammatory cytokine (interleukin 17 [IL-17]) expression in tissues surrounding infected central nervous system catheters. Based on these results, we propose this approach is a valid animal model for further investigations of catheter-associated central nervous system shunt infections.
Figures


References
-
- Baldwin AC, Kielian T. 2004. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J. Neuroimmunol. 151:24–32 - PubMed
-
- Bell MD, et al. 1996. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. Eur. J. Neurosci. 8:1803–1811 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

