Mlp24 (McpX) of Vibrio cholerae implicated in pathogenicity functions as a chemoreceptor for multiple amino acids
- PMID: 22753378
- PMCID: PMC3418727
- DOI: 10.1128/IAI.00039-12
Mlp24 (McpX) of Vibrio cholerae implicated in pathogenicity functions as a chemoreceptor for multiple amino acids
Abstract
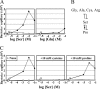
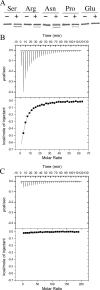
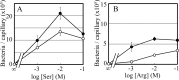
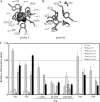
The chemotaxis of Vibrio cholerae, the causative agent of cholera, has been implicated in pathogenicity. The bacterium has more than 40 genes for methyl-accepting chemotaxis protein (MCP)-like proteins (MLPs). In this study, we found that glycine and at least 18 L-amino acids, including serine, arginine, asparagine, and proline, serve as attractants to the classical biotype strain O395N1. Based on the sequence comparison with Vibrio parahaemolyticus, we speculated that at least 17 MLPs of V. cholerae may mediate chemotactic responses. Among them, Mlp24 (previously named McpX) is required for the production of cholera toxin upon mouse infection. mlp24 deletion strains of both classical and El Tor biotypes showed defects in taxis toward several amino acids, which were complemented by the expression of Mlp24. These amino acids enhanced methylation of Mlp24. Serine, arginine, asparagine, and proline were shown to bind directly to the periplasmic fragment of Mlp24. The structural information of its closest homolog, Mlp37, predicts that Mlp24 has two potential ligand-binding pockets per subunit, the membrane distal of which was suggested, by mutational analyses, to be involved in sensing of amino acids. These results suggest that Mlp24 is a chemoreceptor for multiple amino acids, including serine, arginine, and asparagine, which were previously shown to stimulate the expression of several virulence factors, implying that taxis toward a set of amino acids plays critical roles in pathogenicity of V. cholerae.
Figures


Similar articles
-
Calcium Ions Modulate Amino Acid Sensing of the Chemoreceptor Mlp24 of Vibrio cholerae.J Bacteriol. 2019 Apr 9;201(9):e00779-18. doi: 10.1128/JB.00779-18. Print 2019 May 1. J Bacteriol. 2019. PMID: 30745373 Free PMC article.
-
Structural basis of the binding affinity of chemoreceptors Mlp24p and Mlp37p for various amino acids.Biochem Biophys Res Commun. 2020 Feb 26;523(1):233-238. doi: 10.1016/j.bbrc.2019.12.055. Epub 2019 Dec 18. Biochem Biophys Res Commun. 2020. PMID: 31862138
-
Identification of a Vibrio cholerae chemoreceptor that senses taurine and amino acids as attractants.Sci Rep. 2016 Feb 16;6:20866. doi: 10.1038/srep20866. Sci Rep. 2016. PMID: 26878914 Free PMC article.
-
[Vibrios (Vibrio cholerae, V. parahaemolyticus, V. vulnificus)].Nihon Rinsho. 2003 Mar;61 Suppl 3:722-6. Nihon Rinsho. 2003. PMID: 12718054 Review. Japanese. No abstract available.
-
Expression of Vibrio cholerae virulence genes in response to environmental signals.Curr Issues Intest Microbiol. 2002 Sep;3(2):29-38. Curr Issues Intest Microbiol. 2002. PMID: 12400636 Review.
Cited by
-
Structures of the ligand-binding domain of Helicobacter pylori chemoreceptor TlpA.Protein Sci. 2018 Nov;27(11):1961-1968. doi: 10.1002/pro.3503. Protein Sci. 2018. PMID: 30171638 Free PMC article.
-
The Mechanism of Bidirectional pH Taxis in Bacillus subtilis.J Bacteriol. 2020 Jan 29;202(4):e00491-19. doi: 10.1128/JB.00491-19. Print 2020 Jan 29. J Bacteriol. 2020. PMID: 31685537 Free PMC article.
-
Insights into Chemoreceptor MCP2201-Sensing D-Malate.Int J Mol Sci. 2025 May 20;26(10):4902. doi: 10.3390/ijms26104902. Int J Mol Sci. 2025. PMID: 40430039 Free PMC article.
-
MetR-regulated Vibrio cholerae metabolism is required for virulence.mBio. 2012 Sep 25;3(5):e00236-12. doi: 10.1128/mBio.00236-12. Print 2012. mBio. 2012. PMID: 23015737 Free PMC article.
-
How Bacterial Chemoreceptors Evolve Novel Ligand Specificities.mBio. 2020 Jan 21;11(1):e03066-19. doi: 10.1128/mBio.03066-19. mBio. 2020. PMID: 31964737 Free PMC article.
References
-
- Alm RA, Manning PA. 1990. Characterization of the hlyB gene and its role in the production of the El Tor haemolysin of Vibrio cholerae O1. Mol. Microbiol. 4:413–425 - PubMed
-
- Anantharaman V, Aravind L. 2000. Cache—a signaling domain common to animal Ca2+-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem. Sci. 25:535–537 - PubMed
-
- Armitage JP. 1999. Bacterial tactic responses. Adv. Microb. Physiol. 41:229–289 - PubMed
-
- Banerjee R, et al. 2002. Involvement of in vivo induced cheY-4 gene of Vibrio cholerae in motility, early adherence to intestinal epithelial cells and regulation of virulence factors. FEBS Lett. 532:221–226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

