Dopamine- and cAMP-regulated phosphoprotein of 32-kDa (DARPP-32)-dependent activation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1) signaling in experimental parkinsonism
- PMID: 22753408
- PMCID: PMC3431653
- DOI: 10.1074/jbc.M112.388413
Dopamine- and cAMP-regulated phosphoprotein of 32-kDa (DARPP-32)-dependent activation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1) signaling in experimental parkinsonism
Abstract
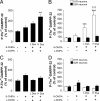
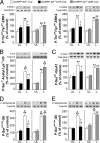
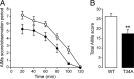
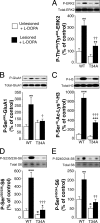
Dyskinesia, a motor complication caused by prolonged administration of the antiparkinsonian drug l-3,4-dihydroxyphenylalanine (l-DOPA), is accompanied by activation of cAMP signaling and hyperphosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32). Here, we show that the abnormal phosphorylation of DARPP-32 occurs specifically in medium spiny neurons (MSNs) expressing dopamine D1 receptors (D1R). Using mice in which DARPP-32 is selectively deleted in D1R-expressing MSNs, we demonstrate that this protein is required for l-DOPA-induced activation of the extracellular signal-regulated protein kinases 1 and 2 and the mammalian target of rapamycin complex 1 (mTORC1) pathways, which are implicated in dyskinesia. We also show that mutation of the phosphorylation site for cAMP-dependent protein kinase on DARPP-32 attenuates l-DOPA-induced dyskinesia and reduces the concomitant activations of ERK and mTORC1 signaling. These studies demonstrate that, in D1R-expressing MSNs, l-DOPA-induced activation of ERK and mTORC1 requires DARPP-32 and indicates the importance of the cAMP/DARPP-32 signaling cascade in dyskinesia.
Figures
References
-
- Kish S. J., Shannak K., Hornykiewicz O. (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 318, 876–880 - PubMed
-
- Marshall J. F., Ungerstedt U. (1977) Supersensitivity to apomorphine following destruction of the ascending dopamine neurons. Quantification using the rotational model. Eur. J. Pharmacol. 41, 361–367 - PubMed
-
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr., Sibley D. R. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432 - PubMed
-
- Guigoni C., Aubert I., Li Q., Gurevich V. V., Benovic J. L., Ferry S., Mach U., Stark H., Leriche L., Håkansson K., Bioulac B. H., Gross C. E., Sokoloff P., Fisone G., Gurevich E. V., Bloch B., Bezard E. (2005) Pathogenesis of levodopa-induced dyskinesia. Focus on D1 and D3 dopamine receptors. Parkinsonism Relat. Disord. 11, S25–S29 - PubMed
-
- Santini E., Valjent E., Fisone G. (2008) Parkinson disease. Levodopa-induced dyskinesia and signal transduction. FEBS J. 275, 1392–1399 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

