Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources
- PMID: 22753413
- PMCID: PMC3436554
- DOI: 10.1074/jbc.M112.372680
Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources
Abstract
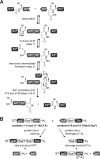
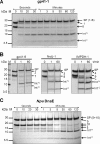
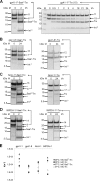
Inteins excise themselves out of precursor proteins by the protein splicing reaction and have emerged as valuable protein engineering tools in numerous and diverse biotechnological applications. Split inteins have recently attracted particular interest because of the opportunities associated with generating a protein from two separate polypeptides and with trans-cleavage applications made possible by split intein mutants. However, natural split inteins are rare and differ greatly in their usefulness with regard to the achievable rates and yields. Here we report the first functional characterization of new split inteins previously identified by bioinformatics from metagenomic sources. The N- and C-terminal fragments of the four inteins gp41-1, gp41-8, NrdJ-1, and IMPDH-1 were prepared as fusion constructs with model proteins. Upon incubation of complementary pairs, we observed trans-splicing reactions with unprecedented rates and yields for all four inteins. Furthermore, no side reactions were detectable, and the precursor constructs were consumed virtually quantitatively. The rate for the gp41-1 intein, the most active intein on all accounts, was k = 1.8 ± 0.5 × 10(-1) s(-1), which is ∼10-fold faster than the rate reported for the Npu DnaE intein and gives rise to completed reactions within 20-30 s. No cross-reactivity in exogenous combinations was observed. Using C1A mutants, all inteins were efficient in the C-terminal cleavage reaction, albeit at lower rates. C-terminal cleavage could be performed under a wide range of reaction conditions and also in the absence of native extein residues flanking the intein. Thus, these inteins hold great potential for splicing and cleavage applications.
Figures

Similar articles
-
Protein trans-splicing of an atypical split intein showing structural flexibility and cross-reactivity.PLoS One. 2012;7(9):e45355. doi: 10.1371/journal.pone.0045355. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024818 Free PMC article.
-
Faster protein splicing with the Nostoc punctiforme DnaE intein using non-native extein residues.J Biol Chem. 2013 Mar 1;288(9):6202-11. doi: 10.1074/jbc.M112.433094. Epub 2013 Jan 10. J Biol Chem. 2013. PMID: 23306197 Free PMC article.
-
Ligation of multiple protein domains using orthogonal inteins with non-native splice junctions.Protein Sci. 2024 Jul;33(7):e5070. doi: 10.1002/pro.5070. Protein Sci. 2024. PMID: 38864750 Free PMC article.
-
[Protein splicing and its application].Sheng Wu Gong Cheng Xue Bao. 2003 Mar;19(2):249-54. Sheng Wu Gong Cheng Xue Bao. 2003. PMID: 15966332 Review. Chinese.
-
Conditional Split Inteins: Adaptable Tools for Programming Protein Functions.Int J Mol Sci. 2025 Jan 11;26(2):586. doi: 10.3390/ijms26020586. Int J Mol Sci. 2025. PMID: 39859302 Free PMC article. Review.
Cited by
-
An expanded library of orthogonal split inteins enables modular multi-peptide assemblies.Nat Commun. 2020 Mar 23;11(1):1529. doi: 10.1038/s41467-020-15272-2. Nat Commun. 2020. PMID: 32251274 Free PMC article.
-
Expanding the SiMPl Plasmid Toolbox for Use with Spectinomycin/Streptomycin.ACS Omega. 2021 May 21;6(22):14148-14153. doi: 10.1021/acsomega.1c00649. eCollection 2021 Jun 8. ACS Omega. 2021. PMID: 34124437 Free PMC article.
-
Near-Infrared Optogenetic Module for Conditional Protein Splicing.J Mol Biol. 2023 Dec 15;435(24):168360. doi: 10.1016/j.jmb.2023.168360. Epub 2023 Nov 8. J Mol Biol. 2023. PMID: 37949312 Free PMC article.
-
Optogenetics with Atomic Precision─A Comprehensive Review of Optical Control of Protein Function through Genetic Code Expansion.Chem Rev. 2025 Feb 26;125(4):1663-1717. doi: 10.1021/acs.chemrev.4c00224. Epub 2025 Feb 10. Chem Rev. 2025. PMID: 39928721 Free PMC article. Review.
-
Intein Zymogens: Conditional Assembly and Splicing of Split Inteins via Targeted Proteolysis.J Am Chem Soc. 2017 Jun 21;139(24):8074-8077. doi: 10.1021/jacs.7b02618. Epub 2017 Jun 7. J Am Chem Soc. 2017. PMID: 28562027 Free PMC article.
References
-
- Hirata R., Ohsumk Y., Nakano A., Kawasaki H., Suzuki K., Anraku Y. (1990) Molecular structure of a gene, VMA1, encoding the catalytic subunit of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J. Biol. Chem. 265, 6726–6733 - PubMed
-
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. (1990) Protein splicing converts the yeast TFP1 gene product to the 69-kDa subunit of the vacuolar H+-adenosine triphosphatase. Science 250, 651–657 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

