Myosin and tropomyosin stabilize the conformation of formin-nucleated actin filaments
- PMID: 22753415
- PMCID: PMC3442522
- DOI: 10.1074/jbc.M112.341230
Myosin and tropomyosin stabilize the conformation of formin-nucleated actin filaments
Abstract
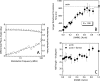
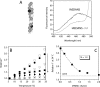
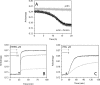
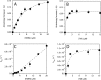
The conformational elasticity of the actin cytoskeleton is essential for its versatile biological functions. Increasing evidence supports that the interplay between the structural and functional properties of actin filaments is finely regulated by actin-binding proteins; however, the underlying mechanisms and biological consequences are not completely understood. Previous studies showed that the binding of formins to the barbed end induces conformational transitions in actin filaments by making them more flexible through long range allosteric interactions. These conformational changes are accompanied by altered functional properties of the filaments. To get insight into the conformational regulation of formin-nucleated actin structures, in the present work we investigated in detail how binding partners of formin-generated actin structures, myosin and tropomyosin, affect the conformation of the formin-nucleated actin filaments using fluorescence spectroscopic approaches. Time-dependent fluorescence anisotropy and temperature-dependent Förster-type resonance energy transfer measurements revealed that heavy meromyosin, similarly to tropomyosin, restores the formin-induced effects and stabilizes the conformation of actin filaments. The stabilizing effect of heavy meromyosin is cooperative. The kinetic analysis revealed that despite the qualitatively similar effects of heavy meromyosin and tropomyosin on the conformational dynamics of actin filaments the mechanisms of the conformational transition are different for the two proteins. Heavy meromyosin stabilizes the formin-nucleated actin filaments in an apparently single step reaction upon binding, whereas the stabilization by tropomyosin occurs after complex formation. These observations support the idea that actin-binding proteins are key elements of the molecular mechanisms that regulate the conformational and functional diversity of actin filaments in living cells.
Figures

References
-
- Le Clainche C., Carlier M. F. (2008) Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88, 489–513 - PubMed
-
- Chhabra E. S., Higgs H. N. (2007) The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, 1110–1121 - PubMed
-
- Miki M., Wahl P., Auchet J. C. (1982) Fluorescence anisotropy of labeled F-actin: influence of divalent cations on the interaction between F-actin and myosin heads. Biochemistry 21, 3661–3665 - PubMed
-
- Hild G., Nyitrai M., Somogyi B. (2002) Intermonomer flexibility of Ca- and Mg-actin filaments at different pH values. Eur. J. Biochem. 269, 842–849 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

