Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors
- PMID: 22753465
- PMCID: PMC3406803
- DOI: 10.1073/pnas.1120068109
Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors
Abstract
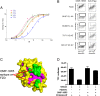
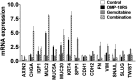
The Wnt/β-catenin pathway, which signals through the Frizzled (Fzd) receptor family and several coreceptors, has long been implicated in cancer. Here we demonstrate a therapeutic approach to targeting the Wnt pathway with a monoclonal antibody, OMP-18R5. This antibody, initially identified by binding to Frizzled 7, interacts with five Fzd receptors through a conserved epitope within the extracellular domain and blocks canonical Wnt signaling induced by multiple Wnt family members. In xenograft studies with minimally passaged human tumors, this antibody inhibits the growth of a range of tumor types, reduces tumor-initiating cell frequency, and exhibits synergistic activity with standard-of-care chemotherapeutic agents.
Conflict of interest statement
Conflict of interest statement: All of the authors are employees of OncoMed Pharmaceuticals, which provided research funding. R.N. is a member of the OncoMed Scientific Advisory Board and holds stock in the company.
Figures




References
-
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. - PubMed
-
- Wang Y. Wnt/Planar cell polarity signaling: A new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. - PubMed
-
- James RG, Conrad WH, Moon RT. Beta-catenin-independent Wnt pathways: Signals, core proteins, and effectors. Methods Mol Biol. 2008;468:131–144. - PubMed
-
- Kohn AD, Moon RT. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

