Molecular dissection of Neurospora Spore killer meiotic drive elements
- PMID: 22753473
- PMCID: PMC3409728
- DOI: 10.1073/pnas.1203267109
Molecular dissection of Neurospora Spore killer meiotic drive elements
Abstract
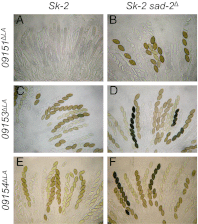
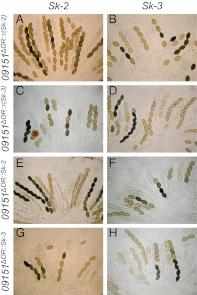
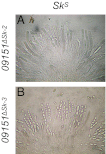
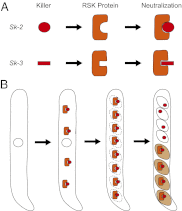
Meiotic drive is a non-Mendelian inheritance phenomenon in which certain selfish genetic elements skew sexual transmission in their own favor. In some cases, progeny or gametes carrying a meiotic drive element can survive preferentially because it causes the death or malfunctioning of those that do not carry it. In Neurospora, meiotic drive can be observed in fungal spore killing. In a cross of Spore killer (Sk) × WT (Sk-sensitive), the ascospores containing the Spore killer allele survive, whereas the ones with the sensitive allele degenerate. Sk-2 and Sk-3 are the most studied meiotic drive elements in Neurospora, and they each theoretically contain two essential components: a killer element and a resistance gene. Here we report the identification and characterization of the Sk resistance gene, rsk (resistant to Spore killer). rsk seems to be a fungal-specific gene, and its deletion in a killer strain leads to self-killing. Sk-2, Sk-3, and naturally resistant isolates all use rsk for resistance. In each killer system, rsk sequences from an Sk strain and a resistant isolate are highly similar, suggesting that they share the same origin. Sk-2, Sk-3, and sensitive rsk alleles differ from each other by their unique indel patterns. Contrary to long-held belief, the killer targets not only late but also early ascospore development. The WT RSK protein is dispensable for ascospore production and is not a target of the spore-killing mechanism. Rather, a resistant version of RSK likely neutralizes the killer element and prevents it from interfering with ascospore development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
A fungal gene reinforces Mendel's laws by counteracting genetic cheating.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):11900-1. doi: 10.1073/pnas.1209748109. Epub 2012 Jul 9. Proc Natl Acad Sci U S A. 2012. PMID: 22778406 Free PMC article. No abstract available.
References
-
- Burt A, Trivers R. Genes in Conflict: The Biology of Selfish Genetic Elements. Cambridge, MA: Belknap Press of Harvard Univ Press; 2006.
-
- Kusano A, Staber C, Chan HY, Ganetzky B. Closing the (Ran)GAP on segregation distortion in Drosophila. Bioessays. 2003;25:108–115. - PubMed
-
- Schimenti J. Segregation distortion of mouse t haplotypes: the molecular basis emerges. Trends Genet. 2000;16:240–243. - PubMed
-
- Raju NB. In: Molecular Biology of Fungal Development. Osiewacz HD, editor. New York: Marcel Dekker; 2002. pp. 275–296.
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

