Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes
- PMID: 22753477
- PMCID: PMC3412008
- DOI: 10.1073/pnas.1209397109
Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes
Abstract
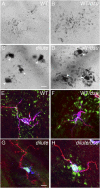
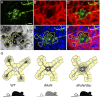
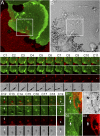
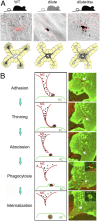
Mammalian pigmentation is driven by the intercellular transfer of pigment-containing melanosomes from the tips of melanocyte dendrites to surrounding keratinocytes. Tip accumulation of melanosomes requires myosin Va, because melanosomes concentrate in the center of melanocytes from myosin Va-null (dilute) mice. This distribution defect results in inefficient melanosome transfer and a dilution of coat color. Dilute mice that simultaneously lack melanoregulin, the product of the dilute suppressor locus, exhibit a nearly complete restoration of coat color, but, surprisingly, melanosomes remain concentrated in the center of their melanocytes. Here we show that dilute/dsu melanocytes, but not dilute melanocytes, readily transfer the melanosomes concentrated in their center to surrounding keratinocytes in situ. Using time-lapse imaging of WT melanocyte/keratinocyte cocultures in which the plasma membranes of the two cells are marked with different colors, we define an intercellular melanosome transfer pathway that involves the shedding by the melanocyte of melanosome-rich packages, which subsequently are phagocytosed by the keratinocyte. Shedding, which occurs primarily at dendritic tips but also from more central regions, involves adhesion to the keratinocyte, thinning behind the forming package, and apparent self-abscission. Finally, we show that shedding from the cell center is sixfold more frequent in cultured dilute/dsu melanocytes than in dilute melanocytes, consistent with the in situ data. Together, these results explain how dsu restores the coat color of dilute mice without restoring intracellular melanosome distribution, indicate that melanoregulin is a negative regulator of melanosome transfer, and provide insight into the mechanism of intercellular melanosome transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

