Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex
- PMID: 22753490
- PMCID: PMC3427089
- DOI: 10.1073/pnas.1205909109
Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex
Abstract
Inhibitory interneurons regulate the responses of cortical circuits. In auditory cortical areas, inhibition from these neurons narrows spectral tuning and shapes response dynamics. Acute disruptions of inhibition expand spectral receptive fields. However, the effects of long-term perturbations of inhibitory circuitry on auditory cortical responses are unknown. We ablated ~30% of dendrite-targeting cortical inhibitory interneurons after the critical period by studying mice with a conditional deletion of Dlx1. Following the loss of interneurons, baseline firing rates rose and tone-evoked responses became less sparse in auditory cortex. However, contrary to acute blockades of inhibition, the sizes of spectral receptive fields were reduced, demonstrating both higher thresholds and narrower bandwidths. Furthermore, long-latency responses at the edge of the receptive field were absent. On the basis of changes in response dynamics, the mechanism for the reduction in receptive field size appears to be a compensatory loss of cortico-cortically (CC) driven responses. Our findings suggest chronic conditions that feature changes in inhibitory circuitry are not likely to be well modeled by acute network manipulations, and compensation may be a critical component of chronic neuronal conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


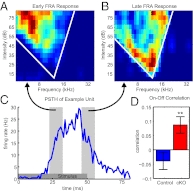

Comment in
-
Consequences of chronic reduction of cortical inhibition.Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13473-4. doi: 10.1073/pnas.1210409109. Epub 2012 Aug 3. Proc Natl Acad Sci U S A. 2012. PMID: 22864915 Free PMC article. No abstract available.
References
-
- Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. - PubMed
-
- Tan AYY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol. 2004;92:630–643. - PubMed
-
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH049428/MH/NIMH NIH HHS/United States
- P50 MH077970/MH/NIMH NIH HHS/United States
- DC02260/DC/NIDCD NIH HHS/United States
- T32 GM007449/GM/NIGMS NIH HHS/United States
- K99 MH085946/MH/NIMH NIH HHS/United States
- MH077970/MH/NIMH NIH HHS/United States
- R37 MH049428/MH/NIMH NIH HHS/United States
- R01 MH049428/MH/NIMH NIH HHS/United States
- MH085946/MH/NIMH NIH HHS/United States
- GM007449/GM/NIGMS NIH HHS/United States
- R01 DC002260/DC/NIDCD NIH HHS/United States
- MH089920/MH/NIMH NIH HHS/United States
- T32 MH089920/MH/NIMH NIH HHS/United States
- R00 MH085946/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

