Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure
- PMID: 22753500
- PMCID: PMC3406816
- DOI: 10.1073/pnas.1116584109
Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure
Abstract
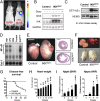
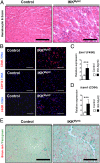
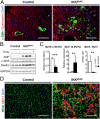
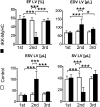
Inflammation is a major factor in heart disease. IκB kinase (IKK) and its downstream target NF-κB are regulators of inflammation and are activated in cardiac disorders, but their precise contributions and targets are unclear. We analyzed IKK/NF-κB function in the heart by a gain-of-function approach, generating an inducible transgenic mouse model with cardiomyocyte-specific expression of constitutively active IKK2. In adult animals, IKK2 activation led to inflammatory dilated cardiomyopathy and heart failure. Transgenic hearts showed infiltration with CD11b(+) cells, fibrosis, fetal reprogramming, and atrophy of myocytes with strong constitutively active IKK2 expression. Upon transgene inactivation, the disease was reversible even at an advanced stage. IKK-induced cardiomyopathy was dependent on NF-κB activation, as in vivo expression of IκBα superrepressor, an inhibitor of NF-κB, prevented the development of disease. Gene expression and proteomic analyses revealed enhanced expression of inflammatory cytokines, and an IFN type I signature with activation of the IFN-stimulated gene 15 (ISG15) pathway. In that respect, IKK-induced cardiomyopathy resembled Coxsackievirus-induced myocarditis, during which the NF-κB and ISG15 pathways were also activated. Vice versa, in cardiomyocytes lacking the regulatory subunit of IKK (IKKγ/NEMO), the induction of ISG15 was attenuated. We conclude that IKK/NF-κB activation in cardiomyocytes is sufficient to cause cardiomyopathy and heart failure by inducing an excessive inflammatory response and myocyte atrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cellular immune reaction in the pancreas is induced by constitutively active IkappaB kinase-2.Gut. 2007 Feb;56(2):227-36. doi: 10.1136/gut.2005.084665. Epub 2006 Jul 26. Gut. 2007. PMID: 16870717 Free PMC article.
-
Sustained, neuron-specific IKK/NF-κB activation generates a selective neuroinflammatory response promoting local neurodegeneration with aging.Mol Neurodegener. 2013 Oct 12;8:40. doi: 10.1186/1750-1326-8-40. Mol Neurodegener. 2013. PMID: 24119288 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Classical NF-κB activation impairs skeletal muscle oxidative phenotype by reducing IKK-α expression.Biochim Biophys Acta. 2014 Feb;1842(2):175-85. doi: 10.1016/j.bbadis.2013.11.001. Epub 2013 Nov 8. Biochim Biophys Acta. 2014. PMID: 24215713
-
Proteins that bind to IKKgamma (NEMO) and down-regulate the activation of NF-kappaB.Biochem Biophys Res Commun. 2010 Jun 4;396(3):585-9. doi: 10.1016/j.bbrc.2010.05.012. Epub 2010 May 10. Biochem Biophys Res Commun. 2010. PMID: 20457134 Review.
Cited by
-
Acute fluoride exposure alters myocardial redox and inflammatory markers in rats.Mol Biol Rep. 2019 Dec;46(6):6155-6164. doi: 10.1007/s11033-019-05050-9. Epub 2019 Sep 3. Mol Biol Rep. 2019. PMID: 31482434
-
Andrographolide attenuates viral myocarditis through interactions with the IL-10/STAT3 and P13K/AKT/NF-κβ signaling pathways.Exp Ther Med. 2018 Sep;16(3):2138-2143. doi: 10.3892/etm.2018.6381. Epub 2018 Jun 29. Exp Ther Med. 2018. PMID: 30186451 Free PMC article.
-
Ginsenoside Rg1 ameliorates cardiac oxidative stress and inflammation in streptozotocin-induced diabetic rats.Diabetes Metab Syndr Obes. 2019 Jul 10;12:1091-1103. doi: 10.2147/DMSO.S208989. eCollection 2019. Diabetes Metab Syndr Obes. 2019. PMID: 31372019 Free PMC article.
-
NF-κB-mediated miR-30b regulation in cardiomyocytes cell death by targeting Bcl-2.Mol Cell Biochem. 2014 Feb;387(1-2):135-41. doi: 10.1007/s11010-013-1878-1. Epub 2013 Nov 1. Mol Cell Biochem. 2014. PMID: 24178239
-
SGLT2 Inhibitors in Aging-Related Cardiovascular Disease: A Review of Potential Mechanisms.Am J Cardiovasc Drugs. 2023 Nov;23(6):641-662. doi: 10.1007/s40256-023-00602-8. Epub 2023 Aug 24. Am J Cardiovasc Drugs. 2023. PMID: 37620652 Review.
References
-
- Dhingra R, Shaw JA, Aviv Y, Kirshenbaum LA. Dichotomous actions of NF-kappaB signaling pathways in heart. J Cardiovasc Transl Res. 2010;3:344–354. - PubMed
-
- Van der Heiden K, Cuhlmann S, Luong A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. - PubMed
-
- Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-κB in the heart: To be or not to NF-κB. Circ Res. 2011;108:1122–1132. - PubMed
-
- Dawn B, et al. Cardiac-specific abrogation of NF- kappa B activation in mice by transdominant expression of a mutant I kappa B alpha. J Mol Cell Cardiol. 2001;33:161–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

