Functional single-cell hybridoma screening using droplet-based microfluidics
- PMID: 22753519
- PMCID: PMC3406880
- DOI: 10.1073/pnas.1204514109
Functional single-cell hybridoma screening using droplet-based microfluidics
Abstract
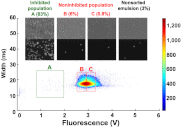
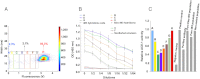
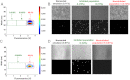
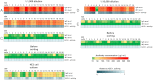
Monoclonal antibodies can specifically bind or even inhibit drug targets and have hence become the fastest growing class of human therapeutics. Although they can be screened for binding affinities at very high throughput using systems such as phage display, screening for functional properties (e.g., the inhibition of a drug target) is much more challenging. Typically these screens require the generation of immortalized hybridoma cells, as well as clonal expansion in microtiter plates over several weeks, and the number of clones that can be assayed is typically no more than a few thousand. We present here a microfluidic platform allowing the functional screening of up to 300,000 individual hybridoma cell clones within less than a day. This approach should also be applicable to nonimmortalized primary B-cells, as no cell proliferation is required: Individual cells are encapsulated into aqueous microdroplets and assayed directly for the release of antibodies inhibiting a drug target based on fluorescence. We used this system to perform a model screen for antibodies that inhibit angiotensin converting enzyme 1, a target for hypertension and congestive heart failure drugs. When cells expressing these antibodies were spiked into an unrelated hybridoma cell population in a ratio of 1:10,000 we observed a 9,400-fold enrichment after fluorescence activated droplet sorting. A wide variance in antibody expression levels at the single-cell level within a single hybridoma line was observed and high expressors could be successfully sorted and recultivated.
Conflict of interest statement
Conflict of interest statement: C.A.M and A.D.G are inventors on patent applications including some of the ideas described in this manuscript.
Figures

References
-
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9:767–774. - PubMed
-
- Walsh G. Biopharmaceutical benchmarks 2010. Nat Biotechnol. 2010;28:917–924. - PubMed
-
- Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–1116. - PubMed
-
- Karsunke XY, et al. Screening and characterization of new monoclonal anti-benzo[a]pyrene antibodies using automated flow-through microarray technology. J Immunol Methods. 2011;371:81–90. - PubMed
-
- Sawyer A, et al. High throughput production of mouse monoclonal antibodies using antigen microarrays. Proteomics. 2005;5:4070–4081. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

