Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats
- PMID: 22753543
- PMCID: PMC3487043
- DOI: 10.1113/jphysiol.2012.232314
Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats
Abstract
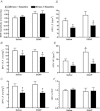
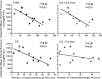
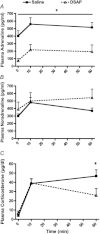
Catecholaminergic neurons within the central nervous system are an integral part of stress-related neurocircuitry, and the nucleus of the solitary tract (NTS) plays a critical role in cardiovascular regulation. We tested the hypothesis that NTS catecholaminergic neurons attenuate psychological stress-induced increases in blood pressure and promote neuroendocrine activation in response to psychological stress.Anti-dopamine-β-hydroxylase antibody conjugated to the neurotoxin saporin (DSAP) or saline vehicle was microinjected into the NTS to lesion catecholaminergic neurons in male Sprague-Dawley rats, and 17 days later the rats were subjected to 60 min of restraint stress for five consecutive days. DSAP treatment significantly enhanced the integrated increase in mean arterial pressure during restraint on the first (800 ± 128 and 1115 ± 116 mmHg (min) for saline- and DSAP-treated rats) and fifth days (655 ± 116 and 1035 ± 113 mmHg (min) for saline- and DSAP-treated rats; P<0.01 for overall effect of DSAP treatment) of restraint. In contrast, after 60 min of restraint plasma corticosterone concentration was significantly lower in DSAP-treated compared with saline-treated rats (25.9 ± 7 compared with 46.8 ± 7 μg dl(-1) for DSAP- and saline-treated rats; P <0.05). DSAP treatment also significantly reduced baseline plasma adrenaline concentration (403 ± 69 compared with 73 ± 29 pg ml(-1) for saline- and DSAP-treated rats), but did not alter the magnitude of the adrenaline response to restraint. The data suggest that NTS catecholaminergic neurons normally inhibit the arterial pressure response, but help maintain the corticosterone response to restraint stress.
Figures




Similar articles
-
Catecholaminergic neurons projecting to the paraventricular nucleus of the hypothalamus are essential for cardiorespiratory adjustments to hypoxia.Am J Physiol Regul Integr Comp Physiol. 2015 Oct;309(7):R721-31. doi: 10.1152/ajpregu.00540.2014. Epub 2015 Jul 8. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26157062 Free PMC article.
-
Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure.Neuroscience. 2008 Oct 28;156(4):1093-102. doi: 10.1016/j.neuroscience.2008.08.011. Epub 2008 Aug 13. Neuroscience. 2008. PMID: 18773942 Free PMC article.
-
A2 noradrenergic lesions prevent renal sympathoinhibition induced by hypernatremia in rats.PLoS One. 2012;7(5):e37587. doi: 10.1371/journal.pone.0037587. Epub 2012 May 21. PLoS One. 2012. PMID: 22629424 Free PMC article.
-
Sudden death following selective neuronal lesions in the rat nucleus tractus solitarii.Auton Neurosci. 2013 Apr;175(1-2):9-16. doi: 10.1016/j.autneu.2012.11.008. Epub 2012 Dec 11. Auton Neurosci. 2013. PMID: 23245583 Free PMC article. Review.
-
Regulation of the stress response in rats by central actions of glucocorticoids.Exp Physiol. 2010 Jan;95(1):26-31. doi: 10.1113/expphysiol.2008.045971. Epub 2009 Sep 11. Exp Physiol. 2010. PMID: 19748967 Free PMC article. Review.
Cited by
-
Corticosterone inhibits vagal afferent glutamate release in the nucleus of the solitary tract via retrograde endocannabinoid signaling.Am J Physiol Cell Physiol. 2020 Dec 1;319(6):C1097-C1106. doi: 10.1152/ajpcell.00190.2020. Epub 2020 Sep 23. Am J Physiol Cell Physiol. 2020. PMID: 32966126 Free PMC article.
-
Burning Mouth Syndrome and Hypertension: Prevalence, Gender Differences and Association with Pain and Psycho-Social Characteristics-A Case Control Study.Int J Environ Res Public Health. 2023 Jan 22;20(3):2040. doi: 10.3390/ijerph20032040. Int J Environ Res Public Health. 2023. PMID: 36767407 Free PMC article.
-
Knockdown of tyrosine hydroxylase in the nucleus of the solitary tract reduces elevated blood pressure during chronic intermittent hypoxia.Am J Physiol Regul Integr Comp Physiol. 2013 Nov 1;305(9):R1031-9. doi: 10.1152/ajpregu.00260.2013. Epub 2013 Sep 18. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 24049117 Free PMC article.
-
Investigating the effects of artificial baroreflex stimulation on pain perception: A comparative study in no-pain and chronic low back pain individuals.J Physiol. 2024 Dec;602(24):6941-6957. doi: 10.1113/JP286375. Epub 2024 Oct 9. J Physiol. 2024. PMID: 39383258 Free PMC article.
-
Corticolimbic regulation of cardiovascular responses to stress.Physiol Behav. 2017 Apr 1;172:49-59. doi: 10.1016/j.physbeh.2016.10.015. Epub 2016 Oct 25. Physiol Behav. 2017. PMID: 27793557 Free PMC article. Review.
References
-
- Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indicies for heart rate and blood pressure variability and barorelfex function in resting rats and mice. Physiol Meas. 2010;31:1885–1201. - PubMed
-
- Byrum CE, Guyenet PG. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol. 1997;261:529–542. - PubMed
-
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta analysis of prospective evidence. Hypertension. 2010;55:1026–1032. - PubMed
-
- Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Compar Neurol. 1988;274:60–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

