The organic arsenic derivative GMZ27 induces PML-RARα-independent apoptosis in myeloid leukemia cells
- PMID: 22753750
- PMCID: PMC5166574
The organic arsenic derivative GMZ27 induces PML-RARα-independent apoptosis in myeloid leukemia cells
Abstract
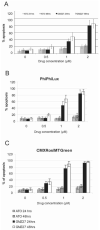
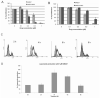
Arsenic trioxide (ATO) is an inorganic arsenic derivative that is very effective against acute promyelocytic leukemia. However, organic arsenic derivatives (OAD) have a more favorable toxicity profile than ATO. We herein characterized dipropil-S-glycerol arsenic (GMZ27), a novel OAD. GMZ27 had potent antiproliferative activity against human acute myeloid leukemia (AML) cell lines that was higher than that of ATO. In contrast to ATO, GMZ27 only marginally induced maturation of leukemia cells and had no effect on the cell cycle. The anti-leukemia activity of GMZ27 against AML cells was independent of the presence of the PML-RARα fusion protein. GMZ27 dissipates mitochondrial transmembrane potential, and induces cleavage of caspase 9 and activation of caspase 3 without altering the expression levels of (BCL-2), BAX and BCL-xl. GMZ27 induces the formation of intracellular superoxide, a reactive oxygen species (ROS) which plays a major role in the antileukemia activity of this OAD. In addition to ROS generation, GMZ27 concomitantly reduces intracellular glutathione which markedly weakens the cellular antioxidant capacity, thus enhancing the detrimental intracellular effects of ROS production. These results indicate that GMZ27 induces apoptosis in AML cells in a PML-RARα-independent fashion, through the induction of ROS production. This activity provides the rationale for the testing of GMZ27 in patients with AML.
Figures


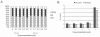


Similar articles
-
MER1, a novel organic arsenic derivative, has potent PML-RARalpha-independent cytotoxic activity against leukemia cells.Invest New Drugs. 2010 Aug;28(4):402-12. doi: 10.1007/s10637-009-9267-z. Epub 2009 May 26. Invest New Drugs. 2010. PMID: 19468689 Free PMC article.
-
Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells.Haematologica. 2007 Mar;92(3):323-31. doi: 10.3324/haematol.10541. Haematologica. 2007. PMID: 17339181
-
Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARalpha independent manner.Blood. 1998 Sep 1;92(5):1497-504. Blood. 1998. PMID: 9716575
-
Mechanistic effects of arsenic trioxide on acute promyelocytic leukemia and other types of leukemias.Cell Biol Int. 2021 Jun;45(6):1148-1157. doi: 10.1002/cbin.11563. Epub 2021 Feb 10. Cell Biol Int. 2021. PMID: 33527587 Review.
-
Interaction between arsenic trioxide and human primary cells: emphasis on human cells of myeloid origin.Inflamm Allergy Drug Targets. 2009 Mar;8(1):21-7. doi: 10.2174/187152809787582516. Inflamm Allergy Drug Targets. 2009. PMID: 19275690 Review.
References
-
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP., Jr Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. - PubMed
-
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutré S, Dahlberg S, Ellison R, Warrell RP., Jr United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. - PubMed
-
- De The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–783. - PubMed
-
- Jeanne M, Lallemand-Breitenbach V, Ferhi O, Koken M, Le Bras M, Duffort S, Peres L, Berthier C, Soilihi H, Raught B, de Thé H. PML/RARA oxidation and arsenic binding initiate the antileukemia response to As2O3. Cancer Cell. 2010;18(1):88–98. - PubMed
-
- Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, de Thé H, Chen SJ, Chen Z. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010;328(5975):240–243. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
